MOMETAMAX SINGLE- gentamicin, posaconazole, and mometasone furoate otic suspension suspension
Mometamax Single by
Drug Labeling and Warnings
Mometamax Single by is a Animal medication manufactured, distributed, or labeled by Merck Sharp & Dohme Corp.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- SAFE HANDLING WARNING
-
DESCRIPTION
DESCRIPTION:
Each milliliter of Mometamax Single contains gentamicin sulfate, equivalent to 8.6 mg gentamicin; 2.6 mg of posaconazole; and mometasone furoate monohydrate, equivalent to 2.1 mg mometasone furoate, in a mineral oil-based system containing a plasticized hydrocarbon gel.
A 0.8 mL dose volume of Mometamax Single delivers approximately 6.88 mg gentamicin, 2.08 mg posaconazole, and 1.68 mg mometasone furoate.
- VETERINARY INDICATIONS
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION:
Mometamax Single should be administered by veterinary personnel. The dose volume is 0.8 mL per affected ear.
Verify the tympanic membrane is intact prior to administration (see Contraindications, Animal Safety Warnings and Precautions).
- 1. Clean and dry the external ear canal before administering the product.
- 2. Shake bottle vigorously for 15 seconds.
- 3. Before first use, unwrap the syringe with the attached adapter.
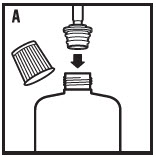
- 4. Remove the cap from the bottle and insert the syringe with the attached adapter by pressing it firmly into the top of the bottle using the attached syringe (see figure A).
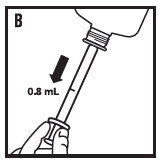
- 5. Invert the bottle and draw up 0.8 mL (see figure B).
- 6. Return the bottle to an upright position and remove the syringe from the adapter. Leave the adapter in place in the bottle for all subsequent uses.
- 7. Replace the cap on the bottle.
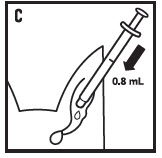
- 8. Place the tip of the syringe at the entrance of the external ear canal and administer the 0.8 mL dose (see figure C). The applied dose will flow into the ear canal.
- 9. Gently massage the base of the ear to ensure distribution of the product throughout the ear canal.
- 10. Restrain the dog post-application to minimize head shaking and to reduce potential splatter of product and accidental eye exposure in people and dogs.
- 11. Use a new syringe for each affected ear. Shake the bottle vigorously (with the cap on the bottle to prevent leakage) for 15 seconds before drawing up each new dose.
- 12. The duration of the effect should last 33 days. Cleaning the ear after dosing may affect product effectiveness.
- 13. Only twenty doses can be accurately withdrawn from the bottle. Discard the bottle 3 months after opening or after 20 doses, whichever comes first.
-
CONTRAINDICATIONS
CONTRAINDICATIONS:
Do not use in dogs with known tympanic membrane perforation (see Animal Safety Warnings and Precautions).
Do not use in dogs with known or suspected hypersensitivity to gentamicin, posaconazole, or mometasone furoate.
-
SPL UNCLASSIFIED SECTION
WARNINGS AND PRECAUTIONS:
USER SAFETY WARNINGS:
Not for human use. Keep this and all drugs out of the reach of children. In case of accidental ingestion by humans, contact a physician immediately.
In case of accidental skin contact, wash area thoroughly with water.
Avoid contact with eyes. If contact with the eyes occurs, flush thoroughly with water for at least 15 minutes. If wearing contact lenses, rinse eyes first then remove the contact lenses and continue to rinse. If symptoms develop, seek medical advice.
Humans with known hypersensitivity to gentamicin, posaconazole, and/or mometasone furoate should avoid handling this product.
-
SPL UNCLASSIFIED SECTION
ANIMAL SAFETY WARNINGS AND PRECAUTIONS:
For otic use in dogs only. Do not use in cats.
Restrain the dog to minimize post-application head shaking. Reducing the potential for splatter of product helps prevent accidental eye exposure in people and dogs (see Dosage and Administration, User Safety Warnings).
The use of Mometamax Single in dogs with perforated tympanic membranes has not been evaluated. The integrity of the tympanic membrane should be confirmed before administering the product. Reevaluate the dog if hearing loss or signs of vestibular dysfunction are observed during treatment.
Do not administer orally.
Use of topical otic corticosteroids has been associated with adrenocortical suppression and iatrogenic hyperadrenocorticism in dogs (see Target Animal Safety).
Use with caution in dogs with impaired renal function. Gentamicin, one of the active ingredients in Mometamax Single, is associated with nephrotoxicity.
The safe use of Mometamax Single in dogs used for breeding purposes, during pregnancy, or during lactation has not been evaluated.
-
ADVERSE REACTIONS
ADVERSE REACTIONS:
The following adverse reactions were reported during the course of a U.S. field study for treatment of otitis externa in dogs treated with Mometamax Single.
Frequency of Adverse Reactions by Treatment
Adverse Reaction Mometamax Single
(N=245)Control
(N=127)Vomiting 21 (8.6%) 4 (3.1%) Decreased appetite 9 (3.7%) 2 (1.6%) Pruritus 8 (3.3%) 2 (1.6%) Ear pruritus 7 (2.9%) 1 (0.8%) Disorientation 3 (1.2%) 0 (0.0%) -
SPL UNCLASSIFIED SECTION
CONTACT INFORMATION:
For technical information or to report a suspected adverse event, please contact Merck Animal Health at 1-800-224-5318 or https://www.merck-animal-health-usa.com. Safety Data Sheets (SDSs) can be found at https://www.merck.com/products/safety-data-sheets/#.
For additional information about reporting adverse drug experiences for animal drugs, contact FDA at 1-888-FDA-VETS or www.fda.gov/reportanimalae.
-
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY:
Mometamax Single is a combination of three active ingredients: gentamicin (antibacterial), posaconazole (antifungal), and mometasone furoate (steroidal anti-inflammatory). Gentamicin is an aminoglycoside bactericidal antibiotic which acts by binding to the 30S ribosomal subunit leading to inhibition of bacterial protein synthesis. Posaconazole is a triazole antifungal which selectively inhibits an enzyme involved in the biosynthesis of ergosterol. Mometasone furoate is a corticosteroid that binds to glucocorticoid receptors resulting in anti-inflammatory activity.
-
MICROBIOLOGY
MICROBIOLOGY:
The compatibility and additive effect of each of the components in Mometamax Single was demonstrated in an in vitro non-interference study which utilized organisms collected from clinical cases of otitis externa in dogs. It determined that gentamicin and posaconazole inhibit the growth of bacteria and yeast commonly associated with otitis externa in dogs. No synergistic or antagonistic effect of the two antimicrobials was demonstrated. The addition of mometasone furoate to the combination did not impair antimicrobial activity.
In a field effectiveness study, each target microbial species (S. pseudintermedius, P. aeruginosa, and M. pachydermatis) was successfully treated in at least 10 or more dogs receiving Mometamax Single (see Effectiveness).
-
SPL UNCLASSIFIED SECTION
EFFECTIVENESS:
In a multicenter, well-controlled, randomized, double-masked field study, Mometamax Single was evaluated against a vehicle control in 372 dogs with otitis externa. Two hundred forty-five dogs were administered Mometamax Single, and 127 dogs were administered the vehicle control. Treatment (0.8 mL) was administered once on Day 0 to the affected ear(s). Prior to treatment, the ear(s) was cleaned with saline. Dogs were evaluated on Days 0, 7, 14, and 33. Blood work and urinalysis were obtained on Day 0 pre-treatment and on Day 33 at study completion. Four clinical signs associated with otitis externa were evaluated: erythema, swelling, ulceration, and exudate. Success was based on clinical improvement on Day 33. Of the 163 dogs included in the effectiveness evaluation, 80.5% of dogs administered Mometamax Single were successfully treated, compared to 19.6% of dogs administered the vehicle control (p<0.0001).
No clinically relevant treatment-related findings were noted in safety parameters.
-
SPL UNCLASSIFIED SECTION
TARGET ANIMAL SAFETY:
Margin of Safety Study: Mometamax Single was administered to 11- to 12-week-old puppies (4 dogs/sex/group) with 1×, 3×, or 5× the labeled dose of 0.8 mL/ear to both ears once every two weeks for a total of 3 doses on Days 1, 15, and 29. The control group received vehicle control on the same dosing schedule. No treatment-related effects on food consumption, body weights, clinical observations, physical examinations, otoscopic examinations, hearing assessments, electrocardiography, ophthalmic examinations, coagulation parameters, serum chemistry, urinalysis, fecal evaluations, or gross pathology were observed. Mometamax Single administration was associated with mild eosinopenia in the 3× and 5× groups and suppression of serum cortisol levels in the 1×, 3×, and 5× groups. On Day 30, two of eight 1× dogs and all 3× and 5× dogs had suppressed baseline cortisol levels. All control and 1× dogs had normal post-ACTH stimulation cortisol response levels. Seven of eight dogs in each of the 3× and 5× groups had suppressed ACTH stimulation responses but remained within the normal reference range.
The low baseline and post-ACTH test cortisol levels correlated with the pathology results of lower mean adrenal weights and mild atrophy of the adrenal cortex in the 3× and 5× groups. Histopathology demonstrated mild atrophy of the external auditory canal epidermis and mild atrophy of the external surface of the tympanic membrane in the 1×, 3×, and 5× groups. One male in the 3× group and one male in the 5× group had a minimal epidermal erosion/ulcer in the external auditory canal of one ear, whereas this finding was not noted in the control or 1× treatment groups.
- STORAGE AND HANDLING
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 24 mL Bottle Carton
Mometamax Single™
(gentamicin, posaconazole, and
mometasone furoate otic suspension)Mometamax Single is indicated for the treatment of otitis
externa associated with susceptible strains of yeast
(Malassezia pachydermatis) and bacteria (Staphylococcus
pseudintermedius and Pseudomonas aeruginosa) in dogs.For Otic Use
in Dogs OnlyDo Not Use in Cats

-
INGREDIENTS AND APPEARANCE
MOMETAMAX SINGLE
gentamicin, posaconazole, and mometasone furoate otic suspension suspensionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0061-5432 Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GENTAMICIN SULFATE (UNII: 8X7386QRLV) (GENTAMICIN - UNII:T6Z9V48IKG) GENTAMICIN 8.6 mg in 1 mL MOMETASONE FUROATE MONOHYDRATE (UNII: MTW0WEG809) (MOMETASONE - UNII:8HR4QJ6DW8) MOMETASONE FUROATE 2.1 mg in 1 mL POSACONAZOLE (UNII: 6TK1G07BHZ) (POSACONAZOLE - UNII:6TK1G07BHZ) POSACONAZOLE 2.6 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0061-5432-01 1 in 1 CARTON 1 24 mL in 1 BOTTLE, DISPENSING Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141600 04/29/2025 Labeler - Merck Sharp & Dohme Corp. (001317601)
Trademark Results [Mometamax Single]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MOMETAMAX SINGLE 97213032 not registered Live/Pending |
Intervet Inc. 2022-01-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.