FLUIDBASE MAX by LABORATORIO GENOVE S.A. FLUIDBASE MAX
FLUIDBASE MAX by
Drug Labeling and Warnings
FLUIDBASE MAX by is a Otc medication manufactured, distributed, or labeled by LABORATORIO GENOVE S.A.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
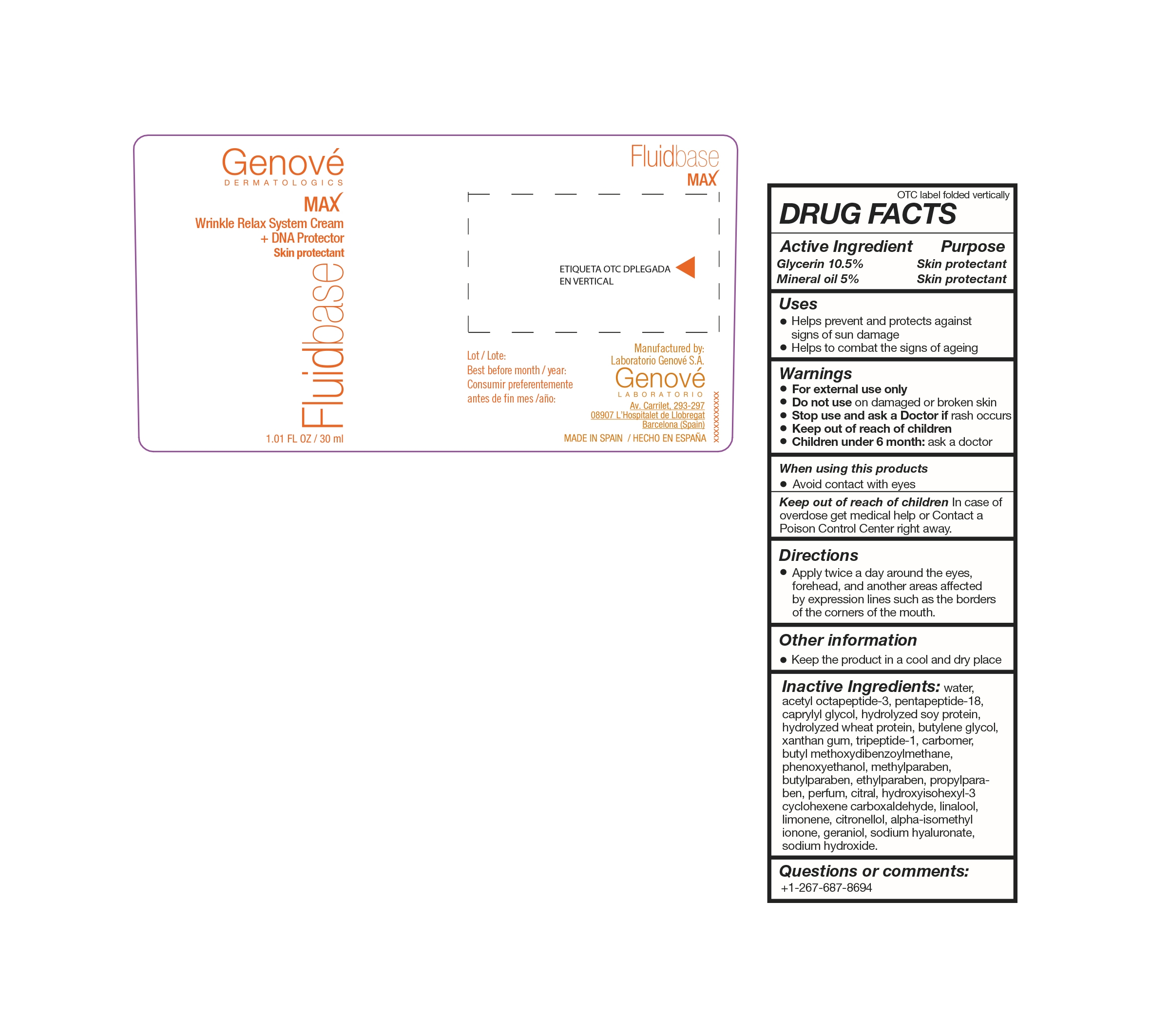
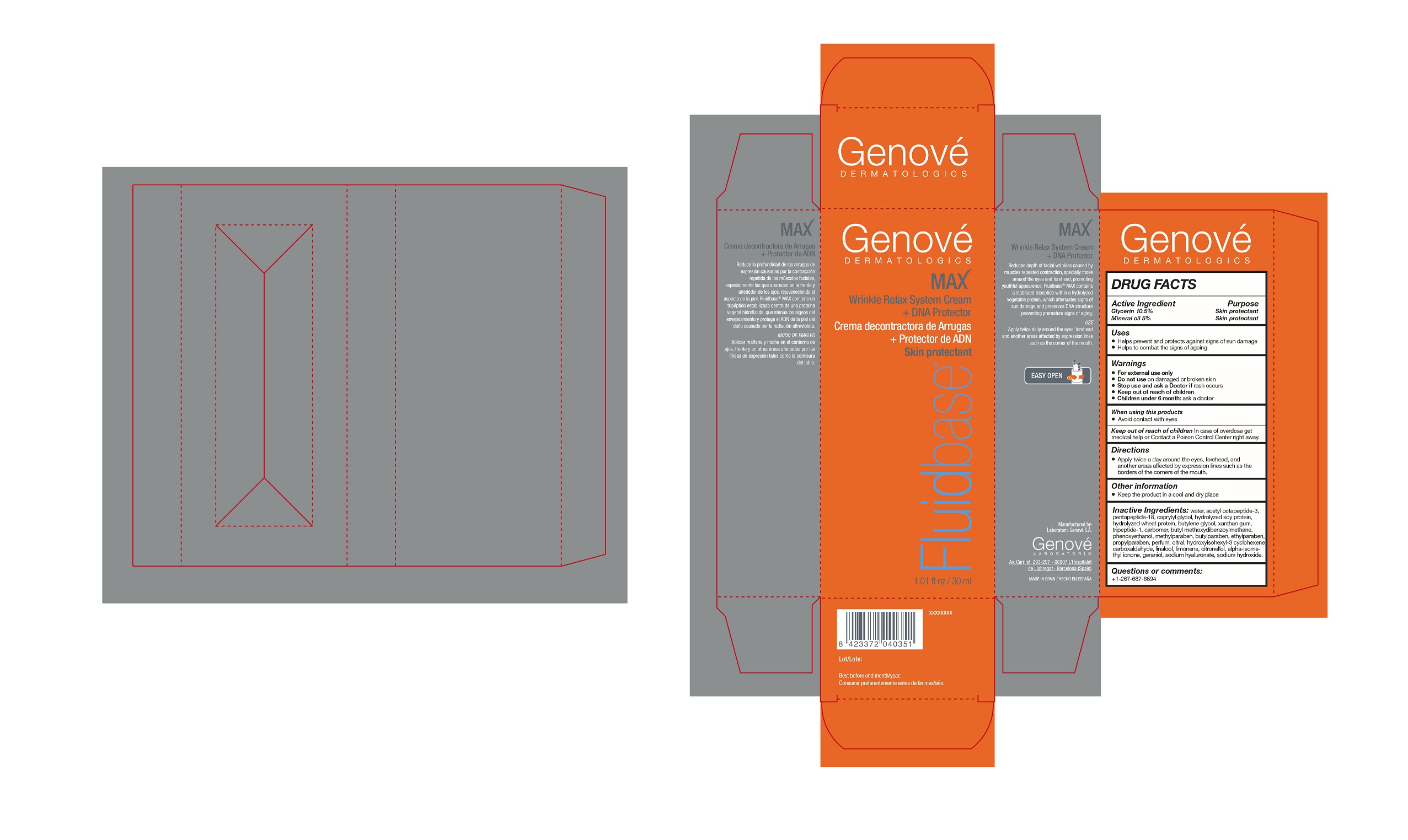
FLUIDBASE MAX- glycerin cream
LABORATORIO GENOVE S.A.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
FLUIDBASE MAX
ACTIVE INGREDIENTS
ACTIVE INGREDIENT PURPOSE
Glycerin 10.5%..........................................................Skin protectant
Mineral Oil 5%...........................................................Skin protectant
WARNINGS
- FOR EXTERNAL USE ONLY
- Do not use on damaged or broken skin
- Stop use and Ask a Doctor if rash occurs;
- Keep out of reach of children
- Children under 6 months: ask a Doctor
KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children: in case of overdose
get medical help or Contact a Poison Control Center
right away.
DIRECTIONS
- Apply twice a day around the eyes, forehead
and another areas affected by expression lines
such as the borders of the corners of the mouth
INDICATIONS
- Apply twice a day around the eyes, forehead
and another areas affected by expression lines
such as the borders of the corners of the mouth
INACTIVE INGREDIENTS
water, acetyl octapeptide-3, leucine, caprylyl glycol, hydrolyzed soy protein, hydrolyzed wheat protein, butylene glycol, xanthan gum, glycyl-isolysine, carbomer, avobenzone, phenoxyethanol, methylparaben, butylparaben, ethylparaben, propylparaben, parfum, citral, hydroxyisohexyl -3- cyclohexene carboxaldehyde, linalool, limonene, citronellol, isomethyl-alpha- ionone, geraniol, hyaluronate sodium, sodium hydroxide
| FLUIDBASE MAX
glycerin cream |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - LABORATORIO GENOVE S.A. (464955435) |
| Registrant - LABORATORIO GENOVE S.A. (464955435) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LABORATORIO GENOVE S.A. | 464955435 | manufacture(70963-001) | |