VETAMEG S- flunixin meglumine injection, solution
Vetameg S by
Drug Labeling and Warnings
Vetameg S by is a Animal medication manufactured, distributed, or labeled by Aspen Veterinary Resources, Norbrook Laboratories Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
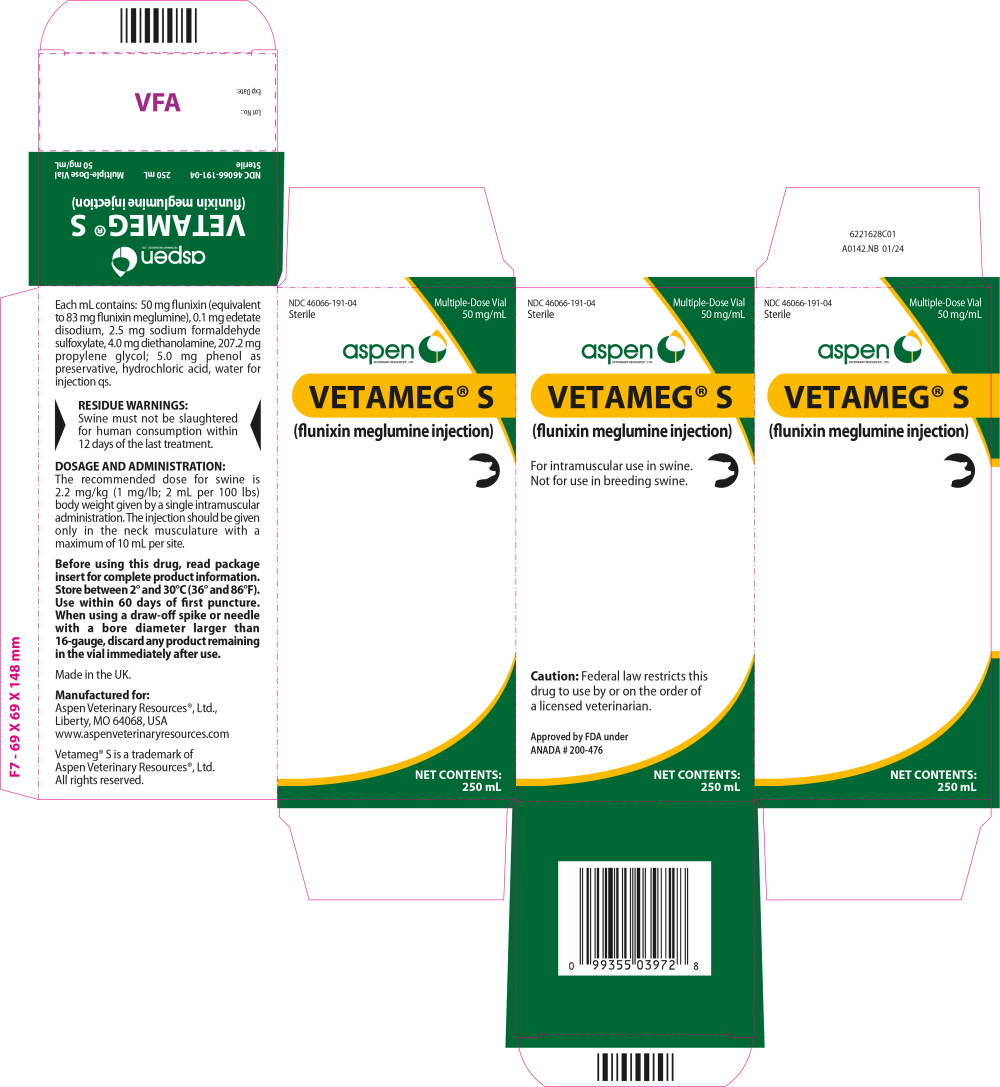
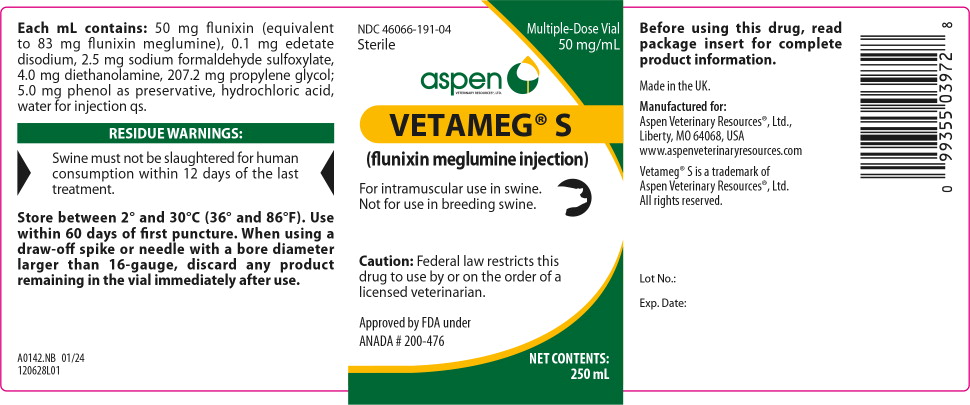
Each milliliter of Vetameg® S (flunixin meglumine injection) contains 50 mg flunixin (equivalent to 83 mg flunixin meglumine), 0.1 mg edetate disodium, 2.5 mg sodium formaldehyde sulfoxylate, 4.0 mg diethanolamine, 207.2 mg propylene glycol; 5.0 mg phenol as preservative, hydrochloric acid, water for injection q.s.
-
CLINICAL PHARMACOLOGY
Flunixin meglumine is a potent non-narcotic, nonsteroidal, analgesic agent with anti-inflammatory and antipyretic activity. It is significantly more potent than pentazocine, meperidine, and codeine as an analgesic in the rat yeast paw test.
Flunixin is known to persist in inflammatory tissues¹ and is associated with anti-inflammatory properties which extend well beyond the period associated with detectable plasma drug concentrations². Therefore, prediction of drug concentrations based upon estimated plasma terminal elimination half-life will likely underestimate both the duration of drug action and the concentration of drug remaining at the site of activity.
The pharmacokinetic profiles were found to follow a 2-compartmental model, although a deep (third) compartment was observed in some animals. The mean terminal elimination half-life (ß half-life) of flunixin after a single intramuscular injection of flunixin injection (2.2 mg/kg) to pigs was between 3 and 4 hours. The mean observed maximum plasma concentration was 2944 ng/mL, achieved at a mean time of approximately 0.4 hours. The mean AUC(0-LOQ) was 6431 ng*hr/mL. Following IM administration of flunixin, quantifiable drug concentration could be measured up to 18 hours post dose. The mean volume of distribution was 2003 mL/kg and the mean total clearance was 390 mL/hr/kg. The mean absolute bioavailability of flunixin following an intramuscular injection in the neck was 87%.
- INDICATION
-
DOSE AND ADMINISTRATION
The recommended dose for swine is 2.2 mg/kg (1 mg/lb; 2 mL per 100 lbs) body weight given by a single intramuscular administration. The injection should be given only in the neck musculature with a maximum of 10 mL per site.
Note: Intramuscular injection may cause local tissue irritation and damage. In an injection-site irritation study, the tissue damage did not resolve in all animals by Day 28 post-injection. This may result in trim loss of edible tissue at slaughter.
- CONTRAINDICATIONS
- RESIDUE WARNINGS
-
PRECAUTIONS
As a class, cyclo-oxygenase inhibitory NSAIDs may be associated with gastrointestinal, renal and hepatic toxicity. Sensitivity to drug-associated adverse events varies with the individual patient. Patients at greatest risk for adverse events are those that are dehydrated, on concomitant diuretic therapy, or those with existing renal, cardiovascular, and/or hepatic dysfunction. Concurrent administration of potentially nephrotoxic drugs should be carefully approached. NSAIDs may inhibit the prostaglandins that maintain normal homeostatic function. Such prostaglandin effects may result in clinically significant disease in patients with underlying or pre-existing disease that has not been previously diagnosed. Since many NSAIDs possess the potential to produce gastrointestinal ulceration, concomitant use of flunixin meglumine with other anti-inflammatory drugs, such as other NSAIDs and corticosteroids, should be avoided.
Not for use in breeding swine. The reproductive effects of flunixin meglumine injection have not been investigated in this class of swine. Intramuscular injection may cause local tissue irritation and damage. In an injection site irritation study, the tissue damage did not resolve in all animals by Day 28 post-injection. This may result in trim loss of edible tissue at slaughter.
-
ADVERSE REACTIONS
Flunixin was mildly irritating at the injection sites. No other flunixin-related changes (adverse reactions) were noted in swine administered a 1 x (2.2 mg/kg; 1.0 mg/lb) dose for 9 days. To report suspected adverse drug events, for technical assistance or to obtain a copy of the Safety Data Sheet (SDS), contact 1-866-591-5777. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
- ANIMAL SAFETY
-
HOW SUPPLIED
Vetameg® S (flunixin meglumine injection), 50 mg/mL, is available in 100 mL and 250 mL multi-dose vials.
Store between 2° and 30°C (36° and 86°F). Use within 60 days of first puncture. When using a draw-off spike or needle with a bore diameter larger than 16-gauge, discard any product remaining in the vial immediately after use.
- Lees P, Higgins AJ. Flunixin inhibits prostaglandin E2 production in equine inflammation. Res Vet Sci. 1984; 37:347-349.
- Odensvik K. Pharmacokinetics of flunixin and its effect onprostaglandin F2α metabolite concentrations after oral and intravenous administration in heifers. J Vet Pharmacal Ther. 1995; 18:254-259.
Approved by FDA under ANADA # 200-476
Made in the UK.
Manufactured for:
Aspen Veterinary Resources®, Ltd.,
Liberty, MO 64068, USA
www.aspenveterinaryresources.comVetameg® S is a trademark of
Aspen Veterinary Resources®, Ltd.
All rights reserved.123628I01
A0141.NB 01/24aspen
VETERINARY RESOURCES,® LTD. -
Principal Display Panel – 250 mL Carton Label
NDC: 46066-191-04
Sterile
Multiple-Dose Vial
50 mg/mL
aspen
VETERINARY RESOURCES,® LTD.VETAMEG® S
(flunixin meglumine injection)
For intramuscular use in swine.
Not for use in breeding swine.
Caution: Federal law restricts this
drug to use by or on the order of
a licensed veterinarian.Approved by FDA under
ANADA # 200-476NET CONTENTS:
250 mL -
Principal Display Panel - 250 mL Vial Label
NDC: 46066-191-04
Sterile
Multiple-Dose Vial
50 mg/mL
aspen
VETERINARY RESOURCES,® LTD.VETAMEG® S
(flunixin meglumine injection)
For intramuscular use in swine.
Not for use in breeding swine.
Caution: Federal law restricts this
drug to use by or on the order of
a licensed veterinarian.Approved by FDA under
ANADA # 200-476NET CONTENTS:
250 mL -
INGREDIENTS AND APPEARANCE
VETAMEG S
flunixin meglumine injection, solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 46066-191 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength flunixin meglumine (UNII: 8Y3JK0JW3U) (flunixin - UNII:356IB1O400) flunixin 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength sodium formaldehyde sulfoxylate (UNII: X4ZGP7K714) 2.5 mg in 1 mL edetate disodium (UNII: 7FLD91C86K) 0.1 mg in 1 mL diethanolamine (UNII: AZE05TDV2V) 4.0 mg in 1 mL propylene glycol (UNII: 6DC9Q167V3) 207.2 mg in 1 mL phenol (UNII: 339NCG44TV) 5.0 mg in 1 mL hydrochloric acid (UNII: QTT17582CB) water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 46066-191-03 1 in 1 CARTON 1 100 mL in 1 VIAL, GLASS 2 NDC: 46066-191-04 1 in 1 CARTON 2 250 mL in 1 VIAL, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200476 03/01/2024 Labeler - Aspen Veterinary Resources (627265361) Registrant - Norbrook Laboratories Limited (214580029) Establishment Name Address ID/FEI Business Operations Norbrook Laboratories Limited 211218325 MANUFACTURE, ANALYSIS, PACK, LABEL
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.