MAFENIDE ACETATE powder, for solution
Mafenide Acetate by
Drug Labeling and Warnings
Mafenide Acetate by is a Prescription medication manufactured, distributed, or labeled by Ingenus Pharmaceuticals, LLC, Novast Laboratories Ltd., Sharp Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Mafenide Acetate, USP is a synthetic antimicrobial agent designated chemically as α-amino-p-toluenesulfonamide monoacetate. It has the following structural formula:
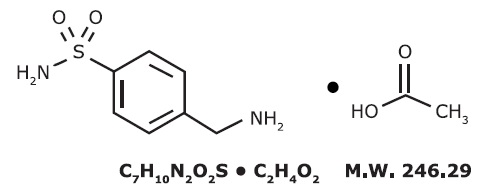
Mafenide Acetate, USP is a white, crystalline powder which is freely soluble in water.
Mafenide Acetate for 5% Topical Solution is provided in packets containing 50 g of sterile Mafenide Acetate to be reconstituted in 1000 mL of Sterile Water for Irrigation, USP or 0.9% Sodium Chloride Irrigation, USP. After mixing, the solution contains 5% w/v of Mafenide Acetate. The solution is an antimicrobial preparation suitable for topical administration. The solution is not for injection. The reconstituted solution may be held up to 28 days after preparation if stored in unopened containers. ONCE A CONTAINER IS OPENED, ANY UNUSED PORTION SHOULD BE DISCARDED AFTER 48 HOURS. Store the reconstituted solution at 20° to 25°C (68° to 77°F). Limited storage periods at 15° to 30°C (59° to 86°F) are acceptable.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
The mechanism of action of Mafenide is not known, but is different from that of the sulfonamides. Mafenide is not antagonized by pABA, serum, pus or tissue exudates, and there is no correlation between bacterial sensitivities to Mafenide and to the sulfonamides. Its activity is not altered by changes in the acidity of the environment. The osmolality of the 5% topical solution is approximately 340 mOsm/kg.
Absorption and Metabolism
Applied topically, Mafenide Acetate diffuses through devascularized areas. Approximately 80% of a Mafenide Acetate dose is delivered to burned tissue over four hours following topical application of the 5% solution. Following application of Mafenide Acetate cream and solution, peak Mafenide concentrations in human burned skin tissue occur at two and four hours, respectively. Peak tissue concentrations are similar following administration of the solution or cream. Once absorbed, Mafenide is rapidly converted to an inactive metabolite (p-carboxybenzene-sulfonamide) which is cleared through the kidneys. Clinical studies have shown that when applied topically to burns as an 11.2% Mafenide Acetate cream, blood levels of the parent drug peaked at 2 hours following application, ranging from 26 to 197 µg/mL for single doses of 14 to 77 g of Mafenide Acetate. Metabolite levels peaked at 3 hours, ranging from 10 to 340 µg/mL. Twenty-four hours after application, combined parent and metabolite blood levels had fallen to pretreatment levels.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
General
Mafenide Acetate and its metabolite, p-carboxybenzene-sulfonamide, inhibit carbonic anhydrase, which may result in metabolic acidosis, usually compensated by hyperventilation. In the presence of impaired renal function, high blood levels of Mafenide Acetate and its metabolite may exaggerate the carbonic anhydrase inhibition. Therefore, close monitoring of acid-base balance is necessary, particularly in patients with extensive second-degree or partial-thickness burns and in those with pulmonary or renal dysfunction. Some burn patients treated with Mafenide Acetate have also been reported to manifest an unexplained syndrome of masked hyperventilation with resulting respiratory alkalosis (slightly alkaline blood pH, low arterial pCO2, and decreased total CO2); change in arterial pO2 is variable. The etiology and significance of these findings are unknown.
Mafenide Acetate should be used with caution in burn patients with acute renal failure.
Fungal colonization may occur concomitantly with reduction of bacterial growth in the burn wound. However, systemic fungal infection through the infected burn wound is rare.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term animal studies have been performed to evaluate the carcinogenic potential of Mafenide Acetate; however, the drug did not induce mutations in L5178Y mouse lymphoma cells at the TK locus.
Animal studies have not been performed to evaluate the potential effects of Mafenide Acetate on fertility.
Pregnancy
Teratogenic Effects. Pregnancy Category C.
A teratology study performed in rats using oral doses of up to 600 mg/kg/day revealed no evidence of harm to the fetus due to Mafenide Acetate. There are no adequate data regarding the potential reproductive toxicity of Mafenide Acetate in a non-rodent species, nor are there adequate and well controlled studies in pregnant women. Mafenide Acetate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether Mafenide Acetate is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Mafenide Acetate, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
-
ADVERSE REACTIONS
In the clinical setting of severe burns, it is often difficult to distinguish between an adverse reaction to Mafenide Acetate and burn sequelae. In a clinical study of pediatric patients with acute burns requiring autografts who received Mafenide Acetate 5% Topical Solution in addition to double antibiotic solution (DAB) wound therapy (neomycin sulfate 40 mg and polymyxin B 200,000 units/liter), the incidence of rash (4.6%) and itching (2.8%) in the group which received Mafenide Acetate 5% Topical Solution was not different from that experienced with (DAB) dressings alone (5.7% and 1.3%, respectively).
From other clinical settings, a single case of bone marrow depression and a single case of an acute attack of porphyria have been reported following therapy with Mafenide Acetate. Fatal hemolytic anemia with disseminated intravascular coagulation, presumably related to a glucose-6-phosphate dehydrogenase deficiency, has been reported following therapy with Mafenide Acetate. The following adverse reactions have been reported with topical Mafenide Acetate therapy:
Dermatologic and Allergic
Pain or burning sensation, rash and pruritus (often localized to the area covered by the wound dressing), erythema, skin maceration from prolonged wet dressings, facial edema, swelling, hives, blisters, eosinophilia.
Respiratory or Metabolic
Tachypnea, hyperventilation, decrease in pCO2, metabolic acidosis, increase in serum chloride.
To report SUSPECTED ADVERSE REACTIONS, please call Ingenus Pharmaceuticals, LLC at 1-877-748-1970 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
- OVERDOSAGE
-
DOSAGE AND ADMINISTRATION
Mafenide Acetate 5% Topical Solution
Directions for Preparation of the Solution
Mafenide Acetate 5% Topical Solution is supplied as a sterile powder and is to be reconstituted with Sterile Water for Irrigation, USP or 0.9% Sodium Chloride Irrigation, USP. Aseptic techniques should be observed during preparation of the solution. Premeasured quantities of 50 g of Mafenide Acetate powder are provided in sterile packets. The entire quantity of Mafenide Acetate pouch should be emptied into a suitable container which contains 1000 mL of Sterile Water for Irrigation, USP or 0.9% Sodium Chloride Irrigation, USP and mixed until completely dissolved. The reconstituted solution may be held up to 28 days after preparation if stored in unopened containers. ONCE A CONTAINER IS OPENED, ANY UNUSED PORTION SHOULD BE DISCARDED AFTER 48 HOURS. Store the reconstituted solution at 20° to 25°C (68° to 77°F). Limited storage periods at 15° to 30°C (59° to 86°F) are acceptable. Not for Injection-For Topical Use Only.
Directions for Use of the Solution
The grafted area should be covered with one layer of fine mesh gauze. An eight-ply burn dressing should be cut to the size of the graft and wetted with Mafenide Acetate 5% Topical Solution using an irrigation syringe and/or irrigation tubing until leaking is noticeable. If irrigation tubing is used, the tubing should be placed over the burn dressing in contact with the wound and covered with a second piece of eight-ply dressing. The irrigation dressing should be secured with a bolster dressing and wrapped as appropriate. The gauze dressing should be kept wet. In clinical studies, this has been accomplished by irrigating with a syringe or injecting the solution into the irrigation tubing every 4 hours or as necessary. If irrigation tubing is not used, the gauze dressing may be moistened every 6-8 hours or as necessary to keep wet.
Wound dressings may be left undisturbed, except for the irrigations, for up to five days. Additional soaks may be initiated until graft take is complete. Maceration of skin may result from wet dressings applied for intervals as short as 24 hours. Treatment is usually continued until autograft vascularization occurs and healing is progressing (typically occurring in about 5 days). Safety and effectiveness have not been established for longer than 5 days for an individual grafting procedure.
If allergic manifestations occur during treatment with Mafenide Acetate 5% Topical Solution, discontinuation of treatment should be considered. If acidosis occurs and becomes difficult to control, particularly in patients with pulmonary dysfunction, discontinuing the soaks with the Mafenide Acetate solution for 24 to 48 hours may aid in restoring acid-base balance (see PRECAUTIONS section). Dressing changes and monitoring the site for bacterial growth during this interruption should be adjusted accordingly.
-
HOW SUPPLIED
Mafenide Acetate for Topical Solution USP, 5% is available in packets (NDC: 50742-310-50) containing 50 g of sterile Mafenide Acetate to be prepared using 1000 mL Sterile Water for Irrigation, USP or 0.9% Sodium Chloride Irrigation, USP. (See DOSAGE AND ADMINISTRATION: Mafenide Acetate for Topical Solution USP, 5%: Directions for Preparation of the Solution.) The packets are supplied as follows:
Carton of five 50 g packets
NDC 50742-310-25 -
Recommended Storage:
Packets - Store PACKETS in a dry place at room temperature 15° to 30°C (59° to 86°F).
Prepared Solution - Store SOLUTION at 20° to 25°C (68° to 77°F) with excursions permitted to 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature.]
The solution may be held for up to 28 days if stored in unopened containers.
ONCE A CONTAINER IS OPENED, ANY UNUSED SOLUTION MUST BE DISCARDED WITHIN 48 HOURS.
Keep this and all medications out of the reach of children.
Manufactured for:
Ingenus Pharmaceuticals, LLC
Orlando, FL 32839-6408
Rev. 08/17 -
PRINCIPAL DISPLAY PANEL
Package Label - Principal Display Panel – 50g Packet
NDC: 50742-310-50
STERILE
Mafenide Acetate
for Topical Solution USP, 5%Not for Injection.
Solution for Topical Use Only.Rx Only
Net Wt. 50 grams sterile powder per packet

-
PRINCIPAL DISPLAY PANEL
Package Label - Principal Display Panel – 5 Packets Carton
NDC: 50742-310-25
STERILE
Mafenide Acetate
for Topical Solution USP, 5%Not for Injection.
Solution for Topical Use Only.Rx Only
5 Packets
Net Wt. 50 grams sterile powder per packet

-
INGREDIENTS AND APPEARANCE
MAFENIDE ACETATE
mafenide acetate powder, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 50742-310 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAFENIDE ACETATE (UNII: RQ6LP6Z0WY) (MAFENIDE - UNII:58447S8P4L) MAFENIDE ACETATE 50 g Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50742-310-25 5 in 1 CARTON 10/22/2018 1 NDC: 50742-310-50 1 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA206716 10/22/2018 Labeler - Ingenus Pharmaceuticals, LLC (833250017) Registrant - Novast Laboratories Ltd. (527695995) Establishment Name Address ID/FEI Business Operations Sharp Corporation 143696495 MANUFACTURE(50742-310)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.