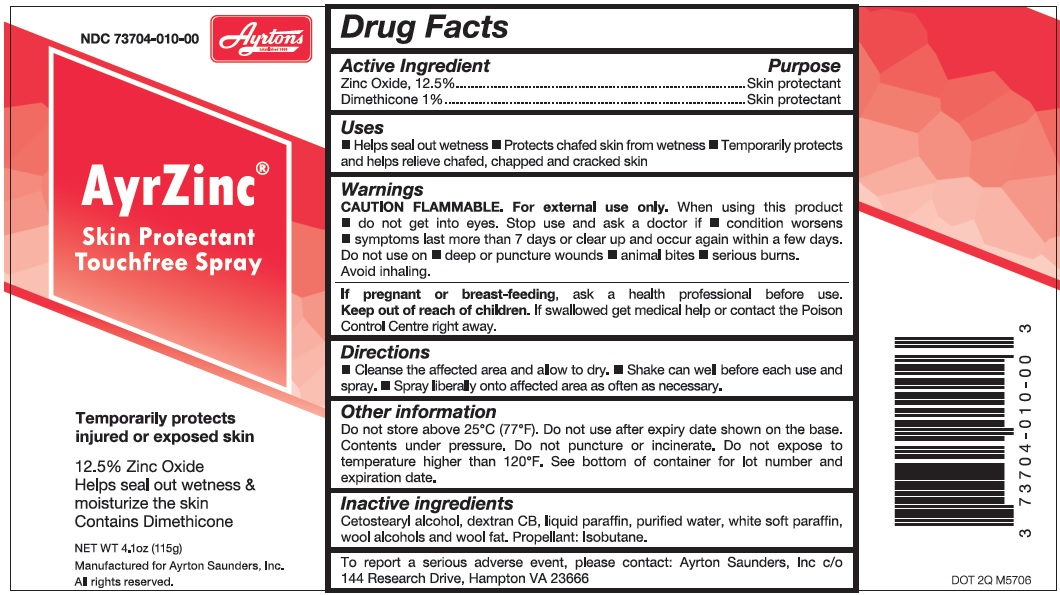
AyrZinc by Ayrton Saunders, INC AyrZinc
AyrZinc by
Drug Labeling and Warnings
AyrZinc by is a Otc medication manufactured, distributed, or labeled by Ayrton Saunders, INC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
AYRZINC- zinc oxide, dimethicone spray, suspension
Ayrton Saunders, INC
----------
AyrZinc
Uses
Helps seal out wetness Protects chafed skin from wetness Temporarily protects and helps relieve chafed, chapped and cracked skin
Warnings
For external use only.
Directions
Cleanse the affected area and allow to dry. Shake can well and spray before each use. Spray liberally onto affected area as often as necessary.
Other information
Do not store above 25°C (77°F). Do not use after expiry date shown on the base. Contents under pressure. Do not puncture or incinerate. Do not store at temperature above 120 °F. See bottom of container for lot number and expiration date.
| AYRZINC
zinc oxide, dimethicone spray, suspension |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Ayrton Saunders, INC (117437357) |
Trademark Results [AyrZinc]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AYRZINC 79205103 5420433 Live/Registered |
Ayrton Saunders Limited 2016-11-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.