FLUIDBASE RETINOL VIT C by LABORATORIO GENOVE S.A. / LABORATORIO GENOVE S A FLUIDBASE RETINOL VIT C
FLUIDBASE RETINOL VIT C by
Drug Labeling and Warnings
FLUIDBASE RETINOL VIT C by is a Otc medication manufactured, distributed, or labeled by LABORATORIO GENOVE S.A., LABORATORIO GENOVE S A. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
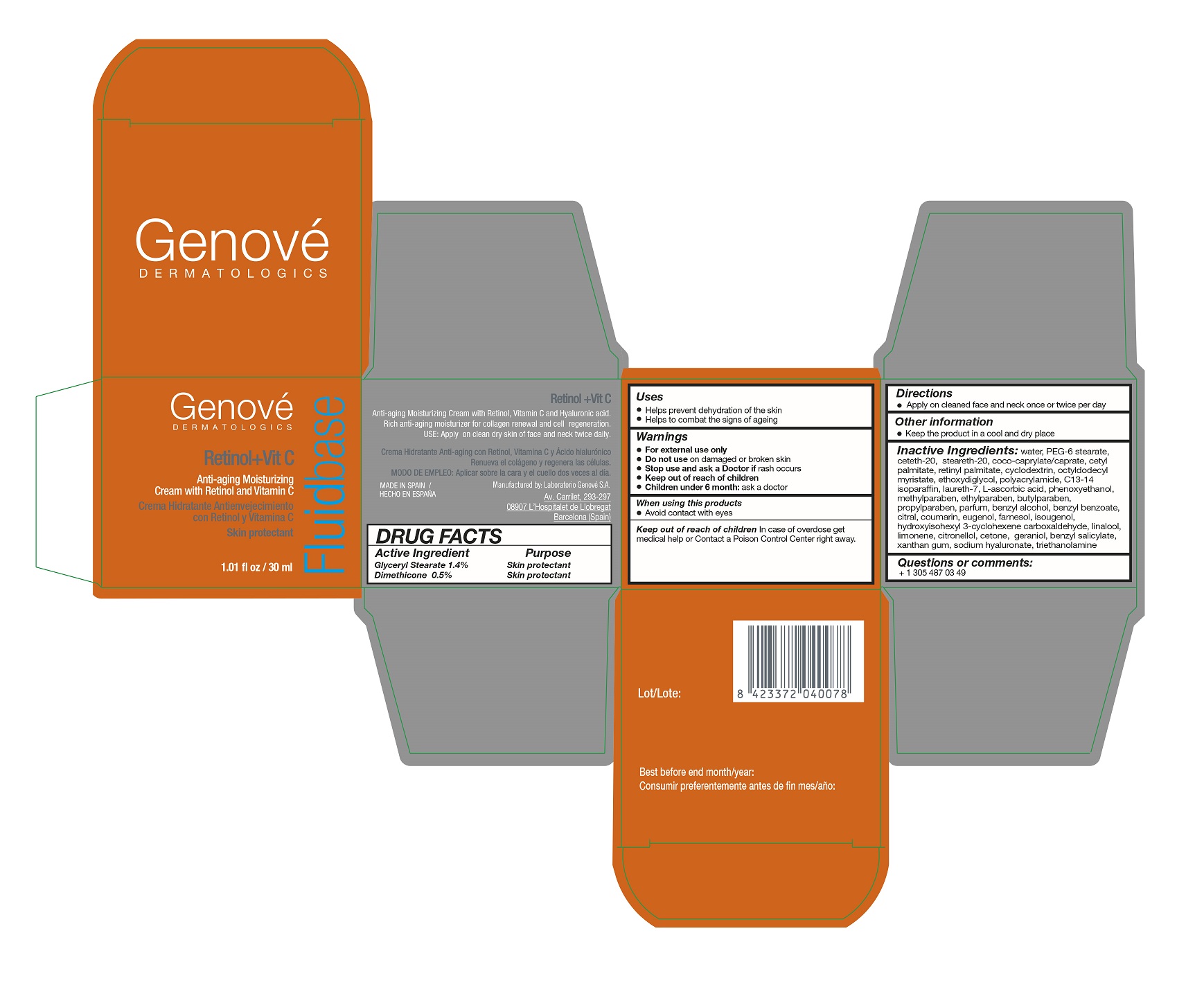
FLUIDBASE RETINOL VIT C- dimethicone lotion
LABORATORIO GENOVE S.A.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
FLUIDBASE RETINOL VIT C
WARNINGS
- For external use only
- Do not use on damaged or broken skin
- Stop use and ask a Doctor is rash occurs
- Keep out of reach of children
- Children under 6 months: ask a doctor
WARNINGS
Keep out of reach of children. In case of overdose
get medical help or contact a Poison Control Center
right away.
WARNINGS
- For external use only
- Do not use on damaged or broken skin
- Stop use and ask a Doctor is rash occurs
- Keep out of reach of children
- Children under 6 months: ask a doctor
INACTIVE INGREDIENTS
water, PEG-6 Stearate, ceteth-20, steareth-20, glyceryl stearate, coco-caprylate/caprate, cetyl palmitate, vitamin A- Palmitate, cyclodextrin, octyldodecyl myristate, diethylene glycol monoethyl ethe, polyacrylamide, C13-14 isoparaffin, laureth-7, ascorbyl glucoside, phenoxyethanol, methylparaben, ethylparaben, butylparaben, propylparaben, perflunafen, benzyl alcohol, benzyl benzoate, citral, coumarin, eugenol, farnesol, isoeugenol, hydroxyisohexyl -3-cyclohexene carboxaldehyde, linalool, limonene, citronellol. alpha isomethyl ionone, geraniol, benzyl salicylate, xanthan gum, hyaluronate sodium, trolamine
| FLUIDBASE RETINOL VIT C
dimethicone lotion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - LABORATORIO GENOVE S.A. (464955435) |
| Registrant - LABORATORIO GENOVE S.A. (464955435) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LABORATORIO GENOVE S A | 464955435 | manufacture(70963-003) | |
