CLEMASTINE FUMARATE syrup
Clemastine Fumarate by
Drug Labeling and Warnings
Clemastine Fumarate by is a Prescription medication manufactured, distributed, or labeled by Lannett Company, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Each teaspoonful (5 mL) of Clemastine Fumarate Syrup for oral administration contains clemastine 0.5 mg (present as clemastine fumarate 0.67 mg). Other ingredients: alcohol 5.5%, flavor, fumaric acid, methylparaben, propylene glycol, propylparaben, purified water, saccharin sodium, sorbitol in a buffered solution. Clemastine fumarate belongs to the benzhydryl ether group of antihistaminic compounds. The chemical name is (+)-(2R)-2-[2-[[(R)-p-Chloro-α-methyl-α-phenylbenzyl]- oxy]ethyl]-1-methylpyrrolidine fumarate and has the following structural formula:
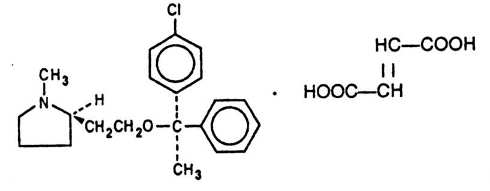
C21H26CINO.C4H4O4 M.W. 459.97
CAS Registration Number 145976-57-9
Clemastine fumarate occurs as a colorless to faintly yellow, practically odorless, crystalline powder. Clemastine Fumarate Syrup has an approximate pH of 6.2.
-
CLINICAL PHARMACOLOGY
Clemastine fumarate is an antihistamine with anticholinergic (drying) and sedative side effects. Antihistamines competitively antagonize various physiological effects of histamine including increased capillary permeability and dilatation, the formation of edema, the "flare" and "itch" response, and gastrointestinal and respiratory smooth muscle constriction. Within the vascular tree, H1-receptor antagonists inhibit both the vasoconstrictor and vasodilator effects of histamine. Depending on the dose, H1- receptor antagonists can produce CNS stimulation or depression. Most antihistamines exhibit central and/or peripheral anticholinergic activity. Antihistamines act by competitively blocking H1-receptor sites. Antihistamines do not pharmacologically antagonize or chemically inactivate histamine, nor do they prevent the release of histamine.
PHARMACOKINETICS
Antihistamines are well-absorbed following oral administration. Chlorpheniramine maleate, clemastine fumarate, and diphenhydramine hydrochloride achieve peak blood levels within 2-5 hours following oral administration. The absorption of antihistamines is often partially delayed by the use of controlled release dosage forms. In these instances, plasma concentrations from identical doses of the immediate and controlled release dosage forms will not be similar. Tissue distribution of the antihistamines in humans has not been established.
Antihistamines appear to be metabolized in the liver chiefly via mono- and didemethylation and glucuronide conjugation. Antihistamine metabolites and small amounts of unchanged drug are excreted in the urine. Small amounts of the drugs may also be excreted in breast milk.
In normal human subjects who received histamine injections over a 24-hour period, the antihistaminic activity of clemastine fumarate reached a peak at 5-7 hours, persisted for 10-12 hours and, in some cases, for as long as 24 hours. Pharmacokinetic studies in man utilizing 3H and 14C labeled compound demonstrates that: clemastine fumarate is rapidly absorbed from the gastrointestinal tract, peak plasma concentrations are attained in 2-4 hours, and urinary excretion is the major mode of elimination.
-
INDICATIONS AND USAGE
Clemastine Fumarate Syrup is indicated for the relief of symptoms associated with allergic rhinitis such as sneezing, rhinorrhea, pruritus and lacrimation. Clemastine Fumarate Syrup is indicated for use in pediatric populations (age 6 years through 12) and adults (see DOSAGE AND ADMINISTRATION).
It should be noted that clemastine fumarate is indicated for the relief of mild uncomplicated allergic skin manifestations of urticaria and angioedema at the 2 mg dosage level only.
-
CONTRAINDICATIONS
Antihistamines are contraindicated in patients hypersensitive to the drug or to other antihistamines of similar chemical structure (see PRECAUTIONS-Drug Interactions). Antihistamines should not be used in newborn or premature Infants. Because of the higher risk of antihistamines for infants generally and for newborns and prematures in particular, antihistamine therapy is contraindicated in nursing mothers (See PRECAUTIONS-Nursing Mothers).
-
WARNINGS
Antihistamines should be used with considerable caution in patients with: narrow angle glaucoma, stenosing peptic ulcer, pyloroduodenal obstruction, symptomatic prostatic hypertrophy, and bladder neck obstruction.
Use with CNS Depressants: Clemastine fumarate has additive effects with alcohol and other CNS depressants (hypnotics, sedatives, tranquilizers, etc.)
Use in Activities Requiring Mental Alertness: Patients should be warned about engaging in activities requiring mental alertness such as driving a car or operating appliances, machinery, etc.
Use in the Elderly (approximately 60 years or older): Antihistamines are more likely to cause dizziness, sedation, and hypotension in elderly patients.
-
PRECAUTIONS
General: Clemastine fumarate should be used with caution in patients with: history of bronchial asthma, increased intraocular pressure, hyperthyroidism, cardiovascular disease, and hypertension. Information for Patients:
- Patients taking antihistamines should receive the following information and instructions: Antihistamines are prescribed to reduce allergic symptoms.
- Patients should be questioned regarding a history of glaucoma, peptic ulcer, urinary retention, or pregnancy before starting antihistamine therapy.
- Patients should be told not to take alcohol, sleeping pills, sedatives, or tranquilizers while taking antihistamines.
- Antihistamines may cause drowsiness, dizziness, dry mouth, blurred vision, weakness, nausea, headache, or nervousness in some patients.
- Patients should avoid driving a car or working with hazardous machinery until they assess the effects of this medicine.
- Patients should be told to store this medicine in a tightly closed container in a dry, cool place away from heat or direct sunlight and out of the reach of children.
Drug Interactions
Additive CNS depression may occur when antihistamines are administered concomitantly with other CNS depressants including barbiturates, tranquilizers, and alcohol. Patients receiving antihistamines should be advised against the concurrent use of other CNS depressant drugs.
Monoamine oxidase (MAO) inhibitors prolong and intensify the anticholinergic effects of antihistamines.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis and Mutagenesis: In a 2-year oral study in the rat at a dose of 84 mg/kg (about 500 times the adult human dose) and an 85-week oral study in the mouse at 206 mg/kg (about 1300 times the adult human dose), clemastine fumarate showed no evidence of carcinogenesis. No mutagenic studies have been conducted with clemastine fumarate.
Impairment of Fertility: Oral doses of clemastine fumarate in the rat produced a decrease in mating ability of the male at 312 times the adult human dose. This effect was not found at 156 times the adult human dose.
Pregnancy
Pregnancy Category B: Oral reproduction studies performed with clemastine fumarate in rats and rabbits at doses up to 312 and 188 times the adult human doses respectively, have revealed no evidence of teratogenic effects.
There are no adequate and well-controlled studies of Clemastine Fumarate Syrup in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used in pregnancy only if clearly needed.
Nursing Mothers
Although quantitative determination of antihistaminic drugs in breast milk have not been reported, qualitative tests have documented the excretion of diphenhydramine, pyrilamine, and tripelennamine in human milk.
Because of the potential for adverse reactions in nursing infants from antihistamines, a decision should be made whether to discontinue nursing or to discontinue the drug.
Pediatric Use
The safety and efficacy of Clemastine Fumarate Syrup has been confirmed in the pediatric population (age 6 years through 12). Safety and dose tolerance studies have confirmed children 6 through 11 years tolerated dosage ranges of 0.75 to 2.25 mg clemastine. In infants and children particularly, antihistamines in overdosage may produce hallucinations, convulsions, and death. Symptoms of antihistamine toxicity in children may include fixed dilated pupils, flushed face, dry mouth, fever, excitation, hallucinations, ataxia, incoordination, athetosis, tonic-clonic convulsions, and postictal depression (see OVERDOSAGE).
-
ADVERSE REACTIONS
The most frequent adverse reactions are underlined: Nervous System: Sedation, sleepiness, dizziness, disturbed coordination, fatigue, confusion, restlessness, excitation, nervousness, tremor, irritability, insomnia, euphoria, paresthesia, blurred vision, diplopia, vertigo, tinnitus, acute labyrinthitis, hysteria, neuritis, convulsions.
Gastrointestinal System: Epigastric distress, anorexia, nausea, vomiting, diarrhea, constipation.
Respiratory System: Thickening of bronchial secretions, tightness of chest and wheezing, nasal stuffiness.
Cardiovascular System: Hypotension, headache, palpitations, tachycardia, extrasystoles.
Hematologic System: Hemolytic anemia, thrombocytopenia, agranulocytosis.
Genitourinary System: Urinary frequency, difficult urination, urinary retention, early menses.
General: Urticaria, drug rash, anaphylactic shock, photosensitivity, excessive perspiration, chills, dryness of mouth, nose and throat.
-
OVERDOSAGE
Antihistamine overdosage reactions may vary from central nervous system depression to stimulation. In children, stimulation predominates initially in a syndrome which may include excitement, hallucinations, ataxia, incoordination, muscle twitching, athetosis, hyperthermia, cyanosis convulsions, tremors, and hyperreflexia followed by postictal depression and cardio respiratory arrest. Convulsions in children may be preceded by mild depression. Dry mouth, fixed dilated pupils, flushing of the face, and fever are common. In adults, CNS depression, ranging from drowsiness to coma, is more common. The convulsant dose of antihistamines lies near the lethal dose. Convulsions indicate a poor prognosis.
In both children and adults, coma and cardiovascular collapse may occur.Deaths are reported especially in infants and children.
There is no specific therapy for acute overdosage with antihistamines. The latent period from ingestion to appearance of toxic effects is characteristically short (1/2-2 hours). General symptomatic and supportive measures should be instituted promptly and maintained for as long as necessary.
Since overdoses of other classes of drugs (i.e., tricyclic antidepressants) may also present anticholinergic symptomatology, appropriate toxicological analysis should be performed as soon as possible to identify the causative agent.
In the conscious patient, vomiting should be induced even though it may have occurred spontaneously. If vomiting cannot be induced, gastric lavage is indicated. Adequate precautions must be taken to protect against aspiration, especially in infants and children. Charcoal slurry or other suitable agents should be instilled into the stomach after vomiting or lavage. Saline cathartics or milk of magnesia may be of additional benefit.
In the unconscious patient, the airway should be secured with a cuffed endotracheal tube before attempting to evacuate the gastric contents. Intensive supportive and nursing care is indicated, as for any comatose patient.
If breathing is significantly impaired, maintenance of an adequate airway and mechanical support of respiration is the most effective means of providing adequate oxygenation. Hypotension is an early sign of impending cardiovascular collapse and should be treated vigorously. Although general supportive measures are important, specific treatment with intravenous infusion of a vasopressor titrated to maintain adequate blood pressure may be necessary.
Do not use with CNS stimulants.
Convulsions should be controlled by careful administration of diazepam or a short-acting barbiturate, repeated as necessary. Physostigmine may also be considered for use in controlling centrally mediated convulsions.
Ice packs and cooling sponge baths, not alcohol, can aid in reducing the fever commonly seen in children.
A more detailed review of antihistamine toxicology and overdose management is available in Gosselin, R.E., et al., "Clinical Toxicology of Commercial Products."
-
DOSAGE AND ADMINISTRATION
DOSAGE SHOULD BE INDIVIDUALIZED ACCORDING TO THE NEEDS AND RESPONSE OF THE PATIENT.
Pediatric: Children aged 6 to 12 years:
For Symptoms Of Allergic Rhinitis -The starting dose is 1 teaspoonful (0.5 mg clemastine) twice daily. Since single doses of up to 2.25 mg clemastine were well tolerated by this age group, dosage may be increased as required, but not to exceed 6 teaspoonfuls daily (3 mg clemastine). For Urticaria and Angioedema -The starting dose is 2 teaspoonfuls (1 mg clemastine) twice daily, not to exceed 6 teaspoonfuls daily (3 mg clemastine).
Adults and Children 12 years and Over:
For Symptoms Of Allergic Rhinitis -The starting dose is 2 teaspoonfuls (1.0 mg clemastine) twice daily. Dosage may be increased as required, but not to exceed 12 teaspoonfuls daily (6 mg clemastine). For Urticaria and Angioedema -The starting dose is 4 teaspoonfuls (2 mg clemastine) twice daily, not to exceed 12 teaspoonfuls daily (6 mg clemastine).
-
HOW SUPPLIED
Clemastine Fumarate Syrup: clemastine 0.5 mg/5 mL (present as clemastine fumarate 0.67 mg/5 mL) is as a clear, colorless liquid with a fruity flavor, and supplied as follows:
120 mL bottle(NDC: 54838-514-40)
473 mL (1 Pint) bottle (NDC: 54838-514-80)
Store below 25°C (77°F). Store in an upright position. Dispense in a tight, light-resistant container.
Manufactured by:
Silarx Pharmaceuticals, Inc.
Spring Valley, NY 10977
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CLEMASTINE FUMARATE
clemastine fumarate syrupProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 54838-514 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLEMASTINE FUMARATE (UNII: 19259EGQ3D) (CLEMASTINE - UNII:95QN29S1ID) CLEMASTINE FUMARATE 0.67 mg in 5 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) FUMARIC ACID (UNII: 88XHZ13131) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SORBITOL (UNII: 506T60A25R) WATER (UNII: 059QF0KO0R) Product Characteristics Color Score Shape Size Flavor PEACH (Peach vanilla) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54838-514-40 120 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/17/1997 2 NDC: 54838-514-80 473 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 12/17/1997 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074884 12/17/1997 Labeler - Lannett Company, Inc. (161630033)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
