CYDECTIN INJECTABLE SOLUTION FOR BEEF AND NONLACTATING DAIRY CATTLE- moxidectin injection, solution
Cydectin by
Drug Labeling and Warnings
Cydectin by is a Animal medication manufactured, distributed, or labeled by Bayer HealthCare, LLC Animal Health Division. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
SPL UNCLASSIFIED SECTION
Injectable Solution for Beef and Nonlactating Dairy Cattle
Antiparasitic
Contains 10 mg moxidectin/mL
Not for use in female dairy cattle 20 months of age or older (including dry dairy cows), veal calves, and calves less than 8 weeks of age.
For Treatment of Infections and Infestations Due to Internal and External Parasites of Cattle
Consult your veterinarian for assistance in the diagnosis, treatment and control of parasitism.
-
PRODUCT DESCRIPTION
CYDECTIN Injectable Solution is a ready-to-use, sterile solution containing 1% moxidectin. Moxidectin is an endectocide in the milbemycin chemical class which shares the distinctive mode of action characteristic of macrocyclic lactones. CYDECTIN Injectable is specially formulated to allow moxidectin to be absorbed from the site of injection and distributed internally to the areas of the body affected by endo- and/or ectoparasitism. Moxidectin binds selectively and with high affinity to glutamate-gated chloride ion channels which are critical to the function of invertebrate nerve and muscle cells. This interferes with neurotransmission resulting in paralysis and elimination of the parasite.
-
INDICATIONS
CYDECTIN Injectable, when administered at the recommended dose level of 0.2 mg/2.2 lb (0.2 mg/kg) body weight, is effective in the treatment and control of the following internal and external parasites of cattle:
Gastrointestinal Roundworms
Lungworms
Ostertagia ostertagi - Adults and L4
Dictyocaulus viviparus - Adults and L4
(including inhibited Larvae)
Cattle Grubs
Haemonchus placei - Adults
Hypoderma bovis
Trichostrongylus axei - Adults and L4
Hypoderma lineatum
Trichostrongylus colubriformis - Adults and L4
Cooperia oncophora - Adults
Mites
Cooperia pectinata - Adults
Psoroptes ovis
Cooperia punctata - Adults and L4
(Psoroptes communis var. bovis)
Cooperia spatulata - Adults
Lice
Cooperia surnabada - Adults and L4
Linognathus vituli
Nematodirus helvetianus - Adults
Solenopotes capillatus
Oesophagostomum radiatum - Adults and L4
Trichuris spp.- Adults
Persistent Activity: CYDECTIN Injectable has been proven to effectively protect cattle from reinfection with Dictyocaulus viviparus and Oesophagostomum radiatum for 42 days after treatment, Haemonchus placei for 35 days after treatment, and Ostertagia ostertagi and Trichostrongylus axei for 14 days after treatment.
Management Considerations for External Parasites: For most effective external parasite control, CYDECTIN Injectable should be administered to all cattle in the herd. Cattle entering the herd following this administration should be treated prior to introduction. Consult your veterinarian or a livestock entomologist for the most appropriate time to administer CYDECTIN Injectable in your location to effectively control external parasites.
-
DOSAGE
The recommended rate of administration for CYDECTIN Injectable is 1 mL for each 110 lb (50 kg) body weight to provide 0.2 mg moxidectin/2.2 lb (0.2 mg/kg) body weight. The table below will assist in the calculation of the appropriate volume of injectable which must be administered based on the weight of animal being treated. Be careful not to overdose animals; estimate animal’s body weight as closely as possible or weigh animals individually.
Weight (lb)
165
220
330
440
550
660
770
880
990
1100
Weight (kg)
75
100
150
200
250
300
350
400
450
500
Dose (mL)
1.5
2.0
3.0
4.0
5.0
6.0
7.0
8.0
9.0
10.0
-
ADMINISTRATION
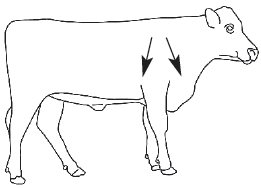
CYDECTIN Injectable should be administered by subcutaneous injection under the loose skin in front of or behind the shoulder (Figure 1). Needles 1/2 to 3/4 inch in length and 16 to 18 gauge are recommended for subcutaneous injections. Use sterile, dry equipment and aseptic procedures when withdrawing and administering CYDECTIN.
Figure 1. Sites for administration of CYDECTIN Injectable
- HUMAN WARNINGS
-
RESIDUE WARNINGS
►Cattle must not be slaughtered for human consumption within 21 days of treatment. This drug product is not approved for use in female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in calves born to these cows. A withdrawal period has not been established for preruminating calves. Do not use in calves to be processed for veal.◄
- ANIMAL SAFETY WARNINGS
- ENVIRONMENTAL WARNINGS
-
PRECAUTIONS
CYDECTIN Injectable has been formulated specifically for subcutaneous injection in cattle and should not be given by other routes of administration. Subcutaneous injection can cause transient local tissue reaction that may result in trim loss of edible tissue at slaughter if animals are slaughtered within 35 days after treatment. This product should not be used in other animal species as severe adverse reactions, including fatalities in dogs, may result.
CYDECTIN Injectable is effective against the migrating stage of cattle grubs (Hypoderma larvae). Treatment with CYDECTIN Injectable during the period when grubs are migrating through vital areas may cause undesirable host-parasite reactions. Killing H. lineatum when they are located in peri-esophageal tissues may cause bloat. Killing H. bovis when they are in the vertebral canal may cause staggering or hindlimb paralysis. Cattle should be treated as soon as possible after heel fly (warble fly) season to avoid this potential problem. Cattle treated with CYDECTIN Injectable at the end of fly season can be retreated during the winter without danger of grub-related reactions. Consult your veterinarian for more information regarding these secondary grub reactions and the correct time to treat with CYDECTIN Injectable.
-
ANIMAL SAFETY
U.S. tolerance and toxicity studies have demonstrated that CYDECTIN Injectable has an adequate margin of safety for use in cattle 8 weeks of age and older. No toxic signs were seen in growing cattle given up to 5 times the recommended dose. Calves as young as 8 weeks of age showed no toxic signs when treated with up to 3 times the recommended dose while nursing from cows concurrently treated with the recommended dose level of CYDECTIN Injectable. Mild, transient ataxia was noted in growing cattle receiving 10 times the recommended dose and in bulls treated at 4.5 times the recommended dose. In breeding animals (bulls and cows in estrous and during early, mid and late pregnancy), treatment with at least 3 times the recommended dose had no effect on breeding performance.
Signs of toxicity include ataxia, excessive salivation, depression, and mydriasis. These signs usually occur within 12 to 48 hours post-treatment.
- STORAGE
- DISPOSAL
- PACKAGE INFORMATION
- SPL UNCLASSIFIED SECTION
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
CYDECTIN INJECTABLE SOLUTION FOR BEEF AND NONLACTATING DAIRY CATTLE
moxidectin injection, solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC: 0859-2414 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength moxidectin (UNII: NGU5H31YO9) (moxidectin - UNII:NGU5H31YO9) moxidectin 10 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0859-2414-01 500 mL in 1 BOTTLE, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141220 08/24/2017 Labeler - Bayer HealthCare, LLC Animal Health Division (152266193)
Trademark Results [Cydectin]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CYDECTIN 75856670 2503548 Live/Registered |
BAYER HEALTHCARE LLC 1999-11-23 |
 CYDECTIN 75726784 2350046 Live/Registered |
BAYER HEALTHCARE LLC 1999-06-11 |
 CYDECTIN 73837625 1605258 Dead/Cancelled |
AMERICAN CYANAMID COMPANY 1989-11-13 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.