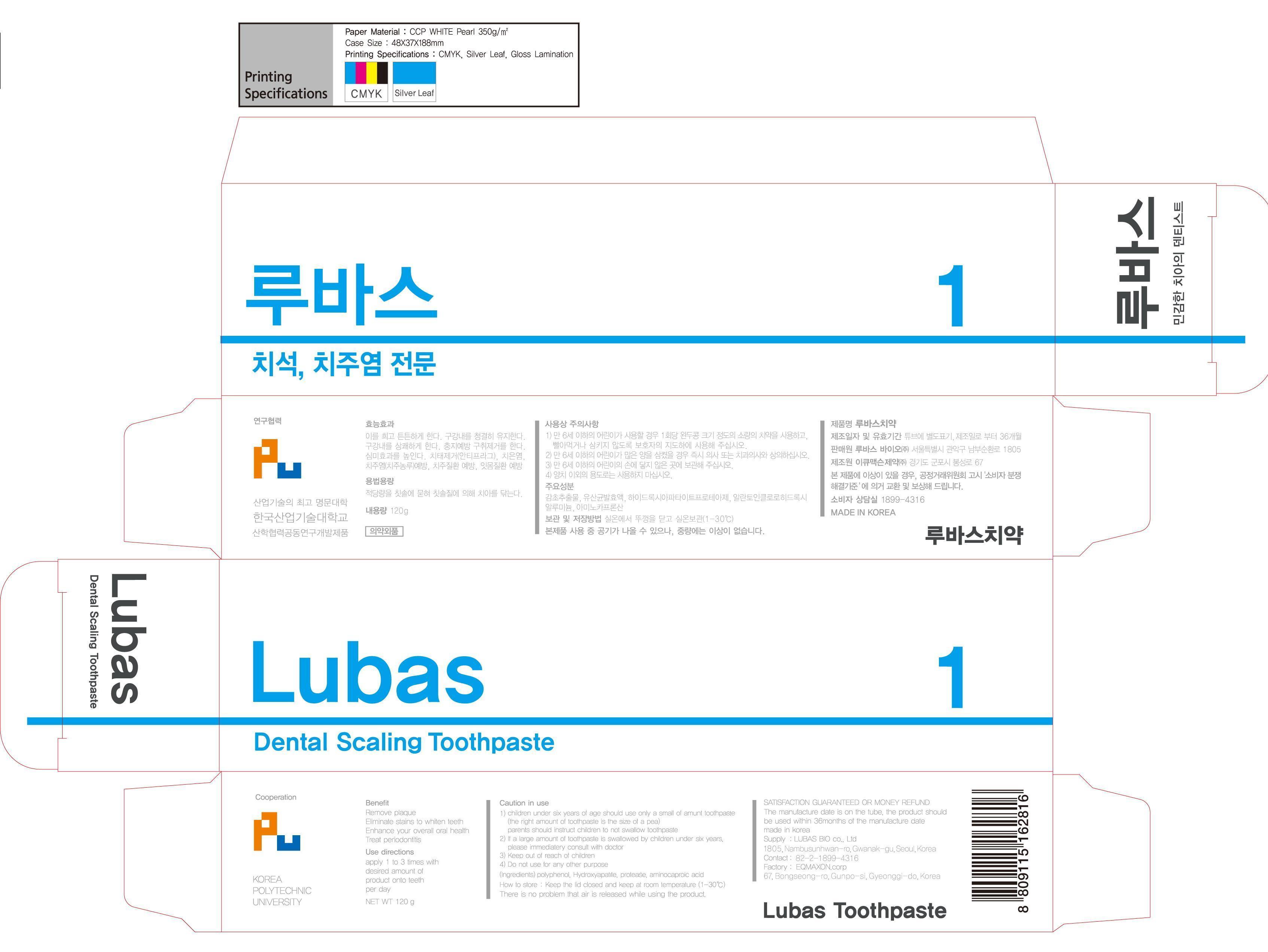
ACTIVE INGREDIENT
silicon dioxide
INACTIVE INGREDIENT
r-aminocapric acid, aluminium chloro hydroxy allantoinate, zeolite, polyedhylene glycol 1500, d-sorbitol solution, glycerine, erythritol, steviol giucoside, xylitol, licorice extract, lactic acid bacteria, sodium carboxymethyl cellulose, methyl parahydroxy benzoate, hydroxylapatite, sodium lauryl sulfate, coolmint flavor, mint flavor, l-menthol, dl-menthol, dionized water
KEEP OUT OF REACH OF CHILDREN
keep out or reach of the children
INDICATIONS & USAGE
apply 1 to 3 times with desired amount of product onto teeth per day
DOSAGE & ADMINISTRATION
for dental use only
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL