METOCLOPRAMIDE HYDROCHLORIDE tablet, orally disintegrating
METOCLOPRAMIDE hydrochloride by
Drug Labeling and Warnings
METOCLOPRAMIDE hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Novel Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use METOCLOPRAMIDE hydrochloride safely and effectively. See full prescribing information for METOCLOPRAMIDE hydrochloride.
METOCLOPRAMIDE hydrochloride (Metoclopramide Hydrochloride) TABLET, ORALLY DISINTEGRATING for ORAL use.
Initial U.S. Approval: 1976WARNING: TARDIVE DYSKINESIA
See full prescribing information for complete boxed warning.
Treatment with metoclopramide can cause tardive dyskinesia, a serious movement disorder that is often irreversible. The risk of developing tardive dyskinesia increases with the duration of treatment and the total cumulative dose.
Metoclopramide therapy should be discontinued in patients who develop signs or symptoms of tardive dyskinesia. There is no known treatment for tardive dyskinesia. In some patients, symptoms may lessen or resolve after metoclopramide treatment is stopped.
Treatment with metoclopramide for longer than 12 weeks should be avoided in all but rare cases where therapeutic benefit is thought to outweigh the risk of developing tardive dyskinesia. (5.1)
INDICATIONS AND USAGE
Metoclopramide Orally Disintegrating Tablets are a dopamine receptor antagonist indicated for:
- Relief of Symptomatic Gastroesophageal Reflux: short-term (4 to 12 weeks) therapy for adults with symptomatic, documented gastroesophageal reflux who fail to respond to conventional therapy (1.1)
- Diabetic Gastroparesis (Diabetic Gastric Stasis): the relief of symptoms in adults associated with acute and recurrent diabetic gastroparesis (gastric stasis) (1.2)
Important Limitations:
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Orally Disintegrating Tablets: 5 mg and 10 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most common adverse reactions (>2%) are headache, nausea, vomiting, fatigue, and somnolence (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Novel Laboratories, Inc. at 1-866-403-7592 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Anticholinergic drugs : Antagonize effects of metoclopramide (7.1)
- Narcotic analgesic drugs : May increase sedation (7.1)
- Monoamine oxidase inhibitors : May cause hypertensive crisis (due to catecholamine release) (7.2)
- Altered drug absorption : May decrease absorption of drugs from the stomach and increase absorption of drugs from the small bowel (7.3)
- Insulin : Changes in food transit time may require adjustment of insulin dose or timing to avoid hypoglycemia (7.4)
- Antidepressants, Antipsychotics, and Neuroleptics : Concomitant use with metoclopramide is associated with increased risk of tardive dyskinesia and Neuroleptic Malignant Syndrome (7.5)
USE IN SPECIFIC POPULATIONS
- Pediatric Use : The safety and effectiveness of Metoclopramide Hydrochloride Orally Disintegrating Tablets in pediatric patients have not been established (8.4)
- Geriatric Use : Elderly patients may be more sensitive to adverse reactions such as sedation and drug-induced movement disorders. (8.5)
- Impaired Renal Function : Initial dosing may need to be reduced and titrated (8.6).
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 12/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: TARDIVE DYSKINESIA
1 INDICATIONS AND USAGE
1.1 Symptomatic Gastroesophageal Reflux Disease
1.2 Diabetic Gastroparesis (Diabetic Gastric Stasis)
1.3 Important Limitations
2 DOSAGE AND ADMINISTRATION
2.1 Important Instructions for Use
2.2 Symptomatic Gastroesophageal Reflux Disease
2.3 Diabetic Gastroparesis (Diabetic Gastric Stasis)
2.4 Renal Impairment
3 DOSAGE FORMS & STRENGTHS
4 CONTRAINDICATIONS
4.1 Intestinal Obstruction, Hemorrhage, or Perforation
4.2 Pheochromocytoma
4.3 Known Sensitivity or Intolerance
4.4 Epilepsy
4.5 Concomitant Medications with Extrapyramidal Reactions
5 WARNINGS AND PRECAUTIONS
5.1 Tardive Dyskinesia
5.2 Acute Dystonic Reactions, Drug-induced Parkinsonism, and Other Extrapyramidal Symptoms
5.3 Neuroleptic Malignant Syndrome
5.4 Depression
5.5 Hypertension
5.6 Congestive Heart Failure and Ventricular Arrhythmia
5.7 Withdrawal from Metoclopramide
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
7.1 Anticholinergic and Narcotic Analgesic Drugs
7.2 Monoamine Oxidase Inhibitors
7.3 Drug Absorption
7.4 Insulin
7.5 Antidepressants, Antipsychotics, and Neuroleptics
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Other Special Populations
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: TARDIVE DYSKINESIA
Treatment with metoclopramide can cause tardive dyskinesia, a serious movement disorder that is often irreversible. The risk of developing tardive dyskinesia increases with the duration of treatment and the total cumulative dose.
Metoclopramide therapy should be discontinued in patients who develop signs or symptoms of tardive dyskinesia. There is no known treatment for tardive dyskinesia. In some patients, symptoms may lessen or resolve after metoclopramide treatment is stopped.
Treatment with metoclopramide for longer than 12 weeks should be avoided in all but rare cases where therapeutic benefit is thought to outweigh the risk of developing tardive dyskinesia.
-
1 INDICATIONS AND USAGE
1.1 Symptomatic Gastroesophageal Reflux Disease
Metoclopramide Hydrochloride Orally Disintegrating Tablets are indicated as short-term (4 to 12 weeks) therapy for adults with symptomatic, documented gastroesophageal reflux disease (GERD) who fail to respond to conventional therapy.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Instructions for Use
Take on an empty stomach at least 30 minutes before eating since food can decrease the peak concentrations of drug in the bloodstream and/or the time it takes to achieve the maximum drug level in the bloodstream [see Clinical Pharmacology (12.3)]. Do not repeat dose if inadvertently taken with food.
Since the tablet absorbs moisture rapidly, only remove each dose from the packaging just prior to taking. Handle the tablet with dry hands and place on the tongue. If the tablet should break or crumble while handling, discard and remove a new tablet.
Metoclopramide Hydrochloride Orally Disintegrating Tablet disintegrates on the tongue in approximately one minute (with a range of 10 seconds to 14 minutes). Metoclopramide Hydrochloride Orally Disintegrating Tablet is designed to be taken without liquid; however, the effect on the pharmacokinetics of Metoclopramide Hydrochloride Orally Disintegrating Tablets taken with liquid is unknown.
2.2 Symptomatic Gastroesophageal Reflux Disease
For the relief of symptomatic, documented gastroesophageal reflux disease (GERD), therapy should not exceed 12 weeks in duration.
Take 10 mg to 15 mg dose of Metoclopramide Hydrochloride Orally Disintegrating Tablets up to four times daily (e.g., at least 30 minutes before each meal and at bedtime). Doses may vary depending upon the symptoms being treated and the clinical response. If symptoms only occur intermittently or at specific times of the day, metoclopramide may be used in single doses up to 20 mg prior to the symptoms rather than continuous treatment.
Since there is a poor correlation between symptomatic relief and healing of esophageal lesions, any therapy directed at esophageal lesions is best confirmed by endoscopic evaluation. Although experience with the effects of metoclopramide on esophageal erosions and ulcerations is limited, healing was documented in a controlled trial using four times daily therapy at 15 mg/dose. Prolonged treatment (>12 weeks) with metoclopramide should be avoided in all but rare cases where therapeutic benefit is thought to counterbalance the risks to the patient of developing tardive dyskinesia. [see Warnings and Precautions (5.1)]
2.3 Diabetic Gastroparesis (Diabetic Gastric Stasis)
For the relief of symptoms associated with diabetic gastroparesis (diabetic gastric stasis), therapy of two to eight weeks is recommended. Therapy should not exceed 12 weeks in duration.
Take a 10 mg dose of Metoclopramide Hydrochloride Orally Disintegrating Tablets up to four times a day (e.g., at least 30 minutes before each meal and at bedtime).
The initial route of administration should be determined by the severity of the presenting symptoms. If only the earliest manifestations of diabetic gastric stasis are present, oral administration of Metoclopramide Hydrochloride Orally Disintegrating Tablets may be initiated. However, if severe symptoms are present, therapy should begin with metoclopramide injection.
Administration of metoclopramide injection up to 10 days may be required before symptoms subside, at which time oral administration may be instituted. Since diabetic gastric stasis is frequently recurrent, Metoclopramide Hydrochloride Orally Disintegrating Tablets therapy should be reinstituted at the earliest manifestation.
2.4 Renal Impairment
Some patients, such as the elderly or those with impaired kidney function (creatinine clearance below 40 mL/min) may be more sensitive to the therapeutic dose or the adverse effects of metoclopramide. Therefore, these patients should start therapy at a lower dose (approximately half the recommended dosage) and the dose should be titrated according to their overall clinical response and/or adverse event profile. Dialysis is not likely to be an effective method of drug removal in overdose situations.
-
3 DOSAGE FORMS & STRENGTHS
5 mg Tablets: Metoclopramide Hydrochloride Orally Disintegrating Tablets are round, white to off-white, flat faced beveled edge tablet, debossed with 'N' on one side and "581" on the other side.
10 mg Tablets: Metoclopramide Hydrochloride Orally Disintegrating Tablets are round, white to off-white, flat faced beveled edge tablet, debossed with 'N' on one side and "580" on the other side.
-
4 CONTRAINDICATIONS
4.1 Intestinal Obstruction, Hemorrhage, or Perforation
Do not use metoclopramide whenever stimulation of gastrointestinal motility may be dangerous such as in the presence of gastrointestinal hemorrhage, mechanical obstruction, or perforation.
4.2 Pheochromocytoma
Metoclopramide is contraindicated in patients with pheochromocytoma because the drug may precipitate a hypertensive crisis, most likely due to release of catecholamines from the tumor. Such hypertensive crises may be controlled by phentolamine.
4.3 Known Sensitivity or Intolerance
Metoclopramide is contraindicated in patients with known sensitivity or intolerance to the drug.
4.4 Epilepsy
Do not use metoclopramide in patients with epilepsy since the frequency and severity of seizures may be increased.
4.5 Concomitant Medications with Extrapyramidal Reactions
Do not use metoclopramide in patients receiving other drugs which are likely to cause extrapyramidal reactions, since the frequency and severity of extrapyramidal reactions may be increased [see Warnings and Precautions (5.2), Adverse Reactions (6.2) and Drug Interactions (7.5)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Tardive Dyskinesia
[see Boxed Warning]
Treatment with metoclopramide can cause tardive dyskinesia (TD), a potentially irreversible and disfiguring disorder characterized by involuntary movements of the face, tongue, or extremities. The risk of developing tardive dyskinesia increases with the duration of treatment and the total cumulative dose. An analysis of utilization patterns showed that about 20% of patients who used metoclopramide took it for longer than 12 weeks. Treatment with metoclopramide for longer than the recommended 12 weeks should be avoided in all but rare cases where therapeutic benefit is thought to outweigh the risk of developing TD.
Although the risk of developing TD in the general population may be increased among the elderly, women, and diabetics, it is not possible to predict which patients will develop metoclopramide-induced TD. Both the risk of developing TD and the likelihood that TD will become irreversible increase with duration of treatment and total cumulative dose.
Metoclopramide should be discontinued in patients who develop signs or symptoms of TD. There is no known effective treatment for established cases of TD, although in some patients, TD may remit, partially or completely, within several weeks to months after metoclopramide is withdrawn.
Metoclopramide itself may suppress, or partially suppress, the signs of TD, thereby masking the underlying disease process. The effect of this symptomatic suppression upon the long-term course of TD is unknown. Therefore, metoclopramide should not be used for the symptomatic control of TD.
5.2 Acute Dystonic Reactions, Drug-induced Parkinsonism, and Other Extrapyramidal Symptoms
Extrapyramidal symptoms (EPS), manifested primarily as acute dystonic reactions, occur in approximately 1 in 500 patients treated with the usual adult dosages of 30 to 40 mg/day of metoclopramide. These usually are seen during the first 24 to 48 hours of treatment with metoclopramide, occur more frequently in pediatric patients and adult patients less than 30 years of age and are even more frequent at higher doses. These symptoms may include involuntary movements of limbs and facial grimacing, torticollis, oculogyric crisis, rhythmic protrusion of tongue, bulbar type of speech, trismus, or dystonic reactions resembling tetanus. Rarely, dystonic reactions may present as stridor and dyspnea, possibly due to laryngospasm. If these symptoms occur, inject 50 mg diphenhydramine hydrochloride intramuscularly. Benztropine mesylate, 1 to 2 mg intramuscularly, may also be used to reverse these reactions.
Drug-induced Parkinsonism can occur during metoclopramide therapy, more commonly within the first 6 months after beginning treatment, but also after longer periods. Parkinsonian symptoms generally subside within 2 to 3 months following discontinuation of metoclopramide. Patients with a history of Parkinson's disease should be given metoclopramide cautiously, if at all, since such patients can experience exacerbation of Parkinsonian symptoms when taking metoclopramide.
5.3 Neuroleptic Malignant Syndrome
There have been rare reports of an uncommon but potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) associated with metoclopramide. Clinical manifestations of NMS include hyperthermia, muscle rigidity, altered consciousness, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis and cardiac arrhythmias). The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, malignant hyperthermia, drug fever and primary central nervous system (CNS) pathology. The management of NMS should include immediate discontinuation of metoclopramide and other drugs not essential to concurrent therapy; intensive symptomatic treatment and medical monitoring; and, treatment of any concomitant serious medical problems for which specific treatments are available. Bromocriptine and dantrolene sodium have been used in treatment of NMS, but their effectiveness has not been established [see Adverse Reactions (6)].
5.4 Depression
Depression associated with metoclopramide use has occurred in patients with and without a history of depression. Symptoms ranged from mild to severe and included suicidal ideation and suicide. For those patients with a prior history of depression, metoclopramide should only be given if the expected benefits outweigh the potential risks.
5.5 Hypertension
In one study in hypertensive patients, intravenously administered metoclopramide was shown to release catecholamines; hence, caution should be exercised when metoclopramide is used in patients with hypertension. There are also clinical reports of hypertensive crises in some patients with undiagnosed pheochromocytoma, thus any rapid rise in blood pressure associated with Metoclopramide Hydrochloride Orally Disintegrating Tablets use should result in immediate cessation of metoclopramide use in those patients [see Contraindications (4.2)].
5.6 Congestive Heart Failure and Ventricular Arrhythmia
Since metoclopramide produces a transient increase in plasma aldosterone, patients with cirrhosis or congestive heart failure may be at risk of developing fluid retention and volume overload. If these side effects occur at any time in any patients during metoclopramide therapy, the drug should be discontinued.
5.7 Withdrawal from Metoclopramide
Adverse reactions, especially those involving the nervous system, may occur after stopping the use of Metoclopramide Hydrochloride Orally Disintegrating Tablets. A small number of patients may experience withdrawal symptoms after stopping that could include dizziness, nervousness, and/or headaches.
Phenylketonurics:
Phenylalanine is a component of aspartame. Each 5 mg and 10 mg Metoclopramide Hydrochloride Orally Disintegrating Tablets contains 4.7 mg of phenylalanine.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in clinical practice.
A total of 86 subjects entered three studies with Metoclopramide Hydrochloride Orally Disintegrating Tablets; 12 subjects entered a pilot bioavailability study (BA); 44 subjects entered a bioequivalence (BE) study, and 30 subjects entered a food-effect study. The adverse reactions from the BE and food-effect study are summarized in Table 1. The pilot BA study data are not included because it was performed with a formulation different from the Metoclopramide Hydrochloride Orally Disintegrating Tablets formulation.
The adverse experience profile seen with Metoclopramide Hydrochloride Orally Disintegrating Tablets was similar to metoclopramide tablets. Thirty-three (33) adverse reactions were reported after receiving Metoclopramide Hydrochloride Orally Disintegrating Tablets and 30 adverse reactions were reported after receiving metoclopramide tablets.
Table 1: Adverse Reactions in BE and Food-Effect Study in ≥2% of Subjects
Adverse
Reaction
Metoclopramide Hydrochloride Orally
Disintegrating TabletsN1,3 (%)2
Metoclopramide tabletsN1,4
(%)2
Nausea
4 (4.2 %)
4 (5.6 %)
Vomiting
2 (2.1 %)
1 (1.4 %)
Fatigue
2 (2.1 %)
2 (2.8 %)
Headache
5 (5.2 %)
3 (4.2 %)
Somnolence
2 (2.1 %)
2 (2.8 %)
Dizziness
1 (1.0 %)
3 (4.2 %)
1 N = number of subjects that reported adverse reactions
2 Percent (%) occurrence = N divided by number of subjects dosed with respective study drug
3 Number of subjects dosed with Metoclopramide Hydrochloride Orally Disintegrating Tablets: 68 under fasted conditions and 28 under fed conditions.
4 Number of subjects dosed with metoclopramide tablets: 28 under fed conditions and 44 under fasted conditions.
The most frequently reported adverse reactions (greater than 2%) associated with Metoclopramide Hydrochloride Orally Disintegrating Tablets were: nausea, vomiting, fatigue, somnolence and headache. The most frequently reported adverse reactions (greater than 2%) associated with metoclopramide tablets were: nausea, headache, fatigue, somnolence, and dizziness. The combined data from the fasted BE study and the food-effect study did not demonstrate any significant differences in the adverse event profile for Metoclopramide Hydrochloride Orally Disintegrating Tablets compared to metoclopramide tablets.
6.2 Post-Marketing Experience
The following adverse reactions are from the cumulative post-marketing experience with metoclopramide tablets. Since the reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
CNS Effects: Restlessness, drowsiness, fatigue, and lassitude occur in approximately 10% of patients receiving the most commonly prescribed dosage of 10 mg four times a day. Insomnia, headache, confusion, dizziness, or depression with suicidal ideation occurs less frequently. The incidence of drowsiness is greater at higher doses. There are isolated reports of seizures without clear-cut relationship to metoclopramide. Rarely, hallucinations have been reported.
Extrapyramidal Syndromes (EPS):
Acute dystonic reactions, the most common type of EPS associated with metoclopramide, occur in approximately 0.2% of patients (1 in 500) treated with 30 to 40 mg of metoclopramide per day. Symptoms include involuntary movements of limbs, facial grimacing, torticollis, oculogyric crisis, rhythmic protrusion of tongue, bulbar type of speech, trismus, opisthotonus (tetanus-like reactions), and rarely, stridor and dyspnea possibly due to laryngospasm; ordinarily these symptoms are readily reversed by diphenhydramine [see Warnings and Precautions (5.1)].
Drug-induced Parkinsonian-like symptoms may include bradykinesia, tremor, cogwheel rigidity, mask-like facies [see Warnings and Precautions (5.2)].
Tardive dyskinesia is most frequently characterized by involuntary movements of the tongue, face, mouth, or jaw, and sometimes by involuntary movements of the trunk and/or extremities; movements may be choreoathetotic in appearance. Motor restlessness (akathisia) may include inability to sit still, pacing, and foot tapping. These symptoms may disappear spontaneously or respond to a reduction in dosage.
Neuroleptic Malignant Syndrome: Rare occurrences of Neuroleptic Malignant Syndrome (NMS) have been reported [see Warnings and Precautions (5.3)].
Endocrine Disturbances: Galactorrhea, amenorrhea, gynecomastia, and impotence secondary to hyperprolactinemia. Fluid retention secondary to transient elevation of aldosterone.
Cardiovascular: Hypotension, hypertension, supraventricular tachycardia, bradycardia, fluid retention, acute congestive heart failure, possible AV block.
Gastrointestinal: Nausea, bowel disturbances, primarily diarrhea.
Hepatic: Rarely, cases of hepatotoxicity characterized by such findings as jaundice and altered liver function tests, when metoclopramide was administered with other drugs with known hepatotoxic potential.
Renal: Urinary frequency and incontinence.
Hematologic: A few cases of neutropenia, leukopenia, or agranulocytosis, generally without clear-cut relationship to metoclopramide. Methemoglobinemia in adults and especially with overdosage in neonates. Sulfhemoglobinemia in adults.
Allergic Reactions: A few cases of rash, urticaria, or bronchospasm, especially in patients with a history of asthma. Rarely, angioneurotic edema, including glossal or laryngeal edema.
Miscellaneous: Visual disturbances. Porphyria.
-
7 DRUG INTERACTIONS
The effects of metoclopramide on gastrointestinal motility can impact the absorption of other drugs. The known drug-drug interactions are listed below.
7.1 Anticholinergic and Narcotic Analgesic Drugs
The effects of metoclopramide on gastrointestinal motility are antagonized by anticholinergic drugs and narcotic analgesics. Additive sedative effects can occur when metoclopramide is given with alcohol, sedatives, hypnotics, narcotics, or tranquilizers.
7.2 Monoamine Oxidase Inhibitors
Metoclopramide has been shown to release catecholamines in patients with essential hypertension suggesting that it should be used cautiously, if at all, in patients taking monoamine oxidase (MAO) inhibitors.
7.3 Drug Absorption
Absorption of drugs from the stomach may be diminished by metoclopramide (e.g., digoxin), whereas the rate and/or extent of absorption of drugs from the small bowel may be increased (e.g., acetaminophen, tetracycline, levodopa, ethanol, cyclosporine).
7.4 Insulin
Because the action of metoclopramide will hasten the movement of food to the intestines and therefore the rate of absorption, insulin dosage or timing of dosage may require adjustment. Increasing movement of food to the intestines may lead to absorption of less glucose from a meal, hence less glucose in the circulation for a particular dose of administered insulin to act upon, resulting in hypoglycemia.
7.5 Antidepressants, Antipsychotics, and Neuroleptics
Concomitant use of metoclopramide should be avoided in patients taking antidepressants, antipsychotics, and/or neuroleptics that have been associated with extrapyramidal reactions such as tardive dyskinesia or Neuroleptic Malignant Syndrome (NMS) that have occurred in association with metoclopramide [see Warnings and Precautions (5.2), (5.3) and Adverse Reactions (6.2)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects: Pregnancy Category B
Reproduction studies have been performed in rats at oral doses about 6 times the maximum recommended human dose calculated on the basis of surface area, and in rabbits at oral doses about 12 times the maximum recommended human dose calculated on the basis of surface area, and have revealed no evidence of impaired fertility or harm to the fetus due to metoclopramide. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
8.3 Nursing Mothers
Metoclopramide is excreted in human milk. Caution should be exercised when metoclopramide is administered to a nursing mother. Because of the potential for serious adverse reactions from metoclopramide in nursing infants and because of the potential for tumorigenicity (including tumor promoting potential in rats), a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
The safety and effectiveness of Metoclopramide Hydrochloride Orally Disintegrating Tablets in pediatric patients have not been established.
The safety profile of Metoclopramide Hydrochloride Orally Disintegrating Tablets in adults cannot be extrapolated to pediatric patients. Dystonias and other extrapyramidal reactions associated with metoclopramide are more common in the pediatric population than in adults. In addition, neonates have reduced levels of NADH-cytochrome b5 reductase making them more susceptible to methemoglobinemia, a possible side effect of metoclopramide use in neonates.
Pediatric PK
The pharmacodynamics of metoclopramide following oral and intravenous administration in pediatric populations are highly variable and a concentration-effect relationship has not been established. Thus, there are insufficient data to conclude whether the pharmacokinetics of Metoclopramide Hydrochloride Orally Disintegrating Tablets in adults and the pediatric population are similar. Although there are insufficient data to support the efficacy of metoclopramide in pediatric patients with symptomatic gastroesophageal reflux disease (GERD) or cancer chemotherapy-related nausea and vomiting, the pharmacokinetics of metoclopramide have been studied in these patient populations and are summarized as follows.
In an open-label study, six pediatric patients (ranging in age from 3.5 weeks to 5.4 months) with GERD received metoclopramide 0.15 mg/kg oral solution every 6 hours for 10 doses. The mean peak plasma concentration of metoclopramide after the tenth dose was twice the level (56.8 mcg/L) compared to after the first dose (29 mcg/L) indicating drug accumulation with repeated dosing. However, the PK parameters after the tenth dose were comparable to those observed after the first dose for the mean time to reach peak concentrations (2.2 hr); half-life (4.1 hr); clearance (0.67 L/h/kg); and volume of distribution (4.4 L/kg). The youngest patient (3.5 weeks) showed a significantly longer half-life after the first dose (23.1 hr) compared to after the tenth dose (10.3 hr), suggesting the reduced clearance observed at birth may be a reflection of the immature hepatic and renal systems.
8.5 Geriatric Use
Clinical studies of metoclopramide did not include sufficient numbers of subjects aged 65 and over to determine whether elderly subjects respond differently from younger subjects.
The risk of developing drug-induced Parkinsonism due to metoclopramide is dose-related. Geriatric patients should receive the lowest dose that is effective. If drug-induced Parkinsonism symptoms develop in a geriatric patient, Metoclopramide Hydrochloride Orally Disintegrating Tablets should be discontinued. The elderly may be at greater risk for tardive dyskinesia [see Warnings and Precautions (5.1)].
Sedation is a potential adverse event associated with metoclopramide use in the elderly.
Metoclopramide is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. For these reasons, dose selection for an elderly patient should be cautious, starting at the low end of the dosing range, due to the greater frequency of decreased renal function, concomitant disease, or other drug therapy in the elderly. [see Warnings and Precautions (5.4)].
8.6 Other Special Populations
Patients with NADH-cytochrome b5 reductase deficiency are at an increased risk of developing methemoglobinemia and/or sulfhemoglobinemia when metoclopramide is administered. In patients with G6PD deficiency who experience metoclopramide-induced methemoglobinemia, methylene blue treatment is not recommended.
Since metoclopramide is excreted principally through the kidneys, therapy should be initiated at approximately one-half the recommended dose in those patients whose creatinine clearance is below 40 mL/min. Depending upon clinical efficacy and safety considerations, the dosage may be increased or decreased as appropriate. Metoclopramide has been safely used in patients with advanced liver disease whose renal function was normal.
-
10 OVERDOSAGE
Symptoms of overdosage may include drowsiness, disorientation, and extrapyramidal reactions. Anticholinergic or anti-Parkinson drugs or antihistamines with anti-cholinergic properties may be helpful in controlling the extrapyramidal reactions. Symptoms are self-limiting and may disappear within 24 hours.
Hemodialysis removes relatively little metoclopramide, probably because of the small amount of the drug in blood relative to tissues. Similarly, continuous ambulatory peritoneal dialysis does not remove significant amounts of drug. It is unlikely that dosage would need to be adjusted to compensate for losses through dialysis. Dialysis is not likely to be an effective method of drug removal in overdose situations.
Unintentional overdose has been reported in infants and children with the use of metoclopramide oral solution. While there was no consistent pattern to the reports associated with these overdoses, events included seizures, extrapyramidal reactions, and lethargy.
Methemoglobinemia has occurred in premature and full-term neonates who were given overdoses of metoclopramide (1 to 4 mg/kg/day orally, intramuscularly or intravenously for 1 to 3 or more days). Methemoglobinemia can be reversed by the intravenous administration of methylene blue. However, methylene blue may cause hemolytic anemia in patients with G6PD deficiency, which may be fatal.
-
11 DESCRIPTION
Metoclopramide Hydrochloride Orally Disintegrating Tablet is an orally disintegrating tablet formulation of metoclopramide hydrochloride. The 5 mg strength tablets are round, white to off-white, flat faced beveled edge tablet debossed with 'N' on one side and "581" on the other side; it is comprised of 5 mg metoclopramide (as 5.91 mg of metoclopramide hydrochloride). The 10 mg tablets are round, white to off-white, flat faced beveled edge tablet debossed with 'N' on one side and "580" on the other side; it is comprised of 10 mg metoclopramide (as 11.82 mg of metoclopramide hydrochloride).
The active ingredient, metoclopramide hydrochloride, is a white crystalline, odorless substance, freely soluble in water. Chemically, it is 4 amino-5-chloro-N-[2-(diethylamino)ethyl]-2-methoxy benzamide monohydrochloride monohydrate. Its molecular formula is C14H22ClN3O2HClH2O. Its molecular weight is 354.3. The structural formula is shown in Figure 1.
Figure 1
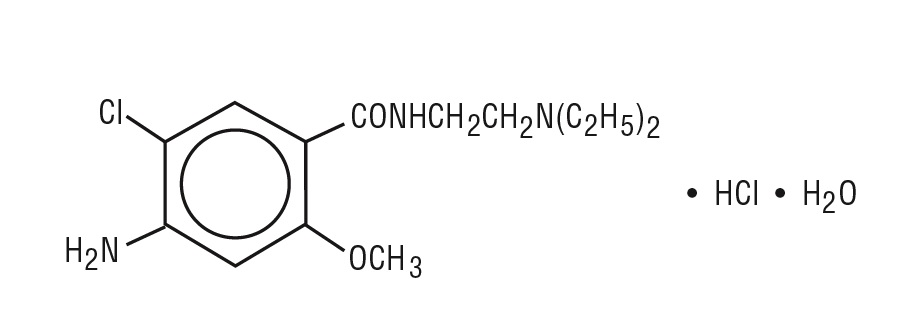
Metoclopramide Hydrochloride Orally Disintegrating Tablets includes the following inactive ingredients: phosphoric acid, mannitol and starch, microcrystalline cellulose, colloidal silicon dioxide, amino methacrylate copolymer, butylated hydroxyanisole, butylated hydroxytoluene, crospovidone, aspartame, N-C mint flavor, magnesium stearate.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Metoclopramide stimulates motility of the upper gastrointestinal tract without stimulating gastric, biliary, or pancreatic secretions. While its mode of action is unclear, it appears to sensitize tissues to the action of acetylcholine. The effect on motility is not dependent on intact vagal innervation, but can be abolished by anticholinergic drugs. Metoclopramide increases the tone and amplitude of gastric (especially antral) contractions, relaxes the pyloric sphincter and the duodenal bulb, and increases peristalsis of the duodenum and jejunum resulting in accelerated gastric emptying and intestinal transit. It increases the resting tone of the lower esophageal sphincter. It has little, if any, effect on the motility of the colon or gallbladder.
The antiemetic properties of metoclopramide appear to be a result of its antagonism of central and peripheral dopamine receptors. Dopamine produces nausea and vomiting by stimulation of the medullary chemoreceptor trigger zone (CTZ), and metoclopramide blocks stimulation of the CTZ by agents like l-dopa or apomorphine, which are known to increase dopamine levels or to possess dopamine-like effects. Metoclopramide also abolishes the slowing of gastric emptying caused by apomorphine. Like the phenothiazines and related drugs, which are also dopamine antagonists, metoclopramide produces sedation and may produce extrapyramidal reactions [see Warnings and Precautions (5.2), (5.3)]. Metoclopramide inhibits the central and peripheral effects of apomorphine, induces release of prolactin, and causes a transient increase in circulating aldosterone levels, which may be associated with transient fluid retention.
12.2 Pharmacodynamics
The onset of pharmacological action of metoclopramide is 30 to 60 minutes following an oral dose; pharmacological effects persist for 1 to 2 hours. In patients with gastroesophageal reflux and low LESP (lower esophageal sphincter pressure), single oral doses of metoclopramide produce dose-related increases in LESP. Effects begin at about 5 mg and increase through 20 mg (the largest dose tested). The increase in LESP from a 5 mg dose lasts about 45 minutes and that of a 20 mg dose lasts between 2 and 3 hours. Increased rate of stomach emptying has been observed with single oral doses of 10 mg.
The principal effect of metoclopramide is on symptoms of post-prandial and daytime heartburn with less observed effect on nocturnal symptoms. If symptoms are confined to particular situations, such as following the evening meal, use of metoclopramide as single doses prior to the provocative situation should be considered, rather than using the drug throughout the day. Healing of esophageal ulcers and erosions has been endoscopically demonstrated at the end of a 12-week trial using doses of 15 mg taken four times a day.
As there is no documented correlation between symptoms and healing of esophageal lesions, patients with documented lesions should be monitored endoscopically. For gastroparesis, the usual manifestations of delayed gastric emptying (e.g., nausea, vomiting, heartburn, persistent fullness after meals, and anorexia) appear to respond within different time intervals.
12.3 Pharmacokinetics
Adult PK of Metoclopramide Hydrochloride Orally Disintegrating Tablets
In a randomized, two-arm, two-way crossover study in 44 healthy adult (male and female) fasted subjects, Metoclopramide Hydrochloride Orally Disintegrating Tablet was bioequivalent to Reglan Tablets.
In a food-effect study with 28 subjects, Metoclopramide Hydrochloride Orally Disintegrating Tablets taken immediately after a high-fat meal had a 17% lower peak blood level than when taken after an overnight fast. The time to peak blood levels increased from about 1.75 hours under fasted conditions to 3 hours when taken immediately after a high-fat meal. The extent of metoclopramide absorbed (area under the curve) was comparable whether Metoclopramide Hydrochloride Orally Disintegrating Tablets was administered with or without food. The clinical effect of the decrease in peak plasma level if Metoclopramide Hydrochloride Orally Disintegrating Tablet is inadvertently taken with food is unknown.
Adult PK of Metoclopramide
Metoclopramide is rapidly and well absorbed. Relative to an intravenous dose of 20 mg, the absolute oral bioavailability of metoclopramide is 80% ± 15.5% as demonstrated in a crossover study of 18 subjects. Peak plasma concentrations occur at about 1 to 2 hr after a single oral dose. Similar time to peak is observed after individual doses at steady state. A single dose study of 12 subjects showed that the area under the drug concentration-time curve increases linearly with doses from 20 to 100 mg (results summarized in Table 2). Peak concentrations increase linearly with dose; time to peak concentrations remains the same; whole body clearance is unchanged; and the elimination rate remains the same. The average elimination half-life in individuals with normal renal function is 5 to 6 hr. Linear kinetic processes adequately describe the absorption and elimination of metoclopramide.
Table 2: Adult Pharmacokinetic Data
Parameter
Value
Vd (L/kg)
~ 3.5
Plasma Protein Binding
~ 30%
T ½
5 to 6 hours
Oral Bioavailability
80% ± 15.5%
Approximately 85% of the radioactivity of an orally administered dose appears in the urine within 72 hr. Of the 85% eliminated in the urine, about half is present as free or conjugated metoclopramide.
The drug is not extensively bound to plasma proteins (about 30%). The whole body volume of distribution is high (about 3.5 L/kg) which suggests extensive distribution of drug to the tissues.
The in vivo disintegration time (time reported between placing the tablet on the tongue and it completely disintegrated into fine particles) was approximately one minute (with a range of 10 seconds to 14 minutes). In the two clinical trials (N = 96) with a mean ± SD being 76.8 ± 110.6 seconds and a median of 53.5 seconds.
Renal impairment affects the clearance of metoclopramide. In a study with patients with varying degrees of renal impairment, a reduction in creatinine clearance was correlated with a reduction in plasma clearance, renal clearance, non-renal clearance, and increase in elimination half-life. The kinetics of metoclopramide in the presence of renal impairment remained linear. The reduction in clearance as a result of renal impairment suggests that reduction of maintenance dosage should be done to avoid drug accumulation.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A 77-week study was conducted in rats with oral doses up to 40 mg/kg/day (about 5 times the maximum recommended human dose on surface area basis). Metoclopramide elevates prolactin levels and the elevation persists during chronic administration. Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin-dependent in vitro, a factor of potential importance if the prescription of metoclopramide is contemplated in a patient with previously detected breast cancer. Although disturbances such as galactorrhea, amenorrhea, gynecomastia, and impotence have been reported with prolactin-elevating drugs, the clinical significance of elevated serum prolactin levels is unknown for most patients. An increase in mammary neoplasms has been found in rodents after chronic administration of prolactin-stimulating neuroleptic drugs and metoclopramide. Neither clinical studies nor epidemiologic studies conducted to date, however, have shown an association between chronic administration of these drugs and mammary tumorigenesis; the available evidence is too limited to be conclusive at this time.
In a rat model for assessing the tumor promotion potential, a two-week oral treatment with metoclopramide at a dose of 260 mg/kg/day (about 35 times the maximum recommended human dose based on body surface area) enhanced the tumorigenic effect of N-nitrosodiethylamine.
Metoclopramide was positive in the in vitro Chinese hamster lung cell / HGPRT forward mutation assay for mutagenic effects and the in vitro human lymphocyte chromosome aberration assay for clastogenic effects. It was negative in the in vitro Ames mutation assay, the in vitro unscheduled DNA synthesis (UDS) assay with rat and human hepatocytes and the in vivo rat micronucleus assay.
Metoclopramide at intramuscular doses up to 20 mg/kg/day (about 3 times the maximum recommended human dose based on body surface area) was found to have no effect on fertility and reproductive performance of male and female rats.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Metoclopramide Hydrochloride Orally Disintegrating Tablets 5 mg strength are round, white to off-white, flat faced beveled edge tablet debossed with 'N' on one side and "581" on the other side; it is comprised of 5 mg metoclopramide (as 5.91 mg of metoclopramide hydrochloride). These are packaged in blister cards as follows:
Box of 10 (1x10) NDC: 40032-581-31
Metoclopramide Hydrochloride Orally Disintegrating Tablets 10 mg are round, white to off-white, flat faced beveled edge tablet debossed with 'N' on one side and "580" on the other side; it is comprised of 10 mg metoclopramide (as 11.82 mg of metoclopramide hydrochloride). These are packaged in blister cards as follows:
Box of 10 (1x10) NDC: 40032-580-31
Tablets should be stored at controlled room temperature, between 20°C and 25°C (68°F and 77°F).
-
17 PATIENT COUNSELING INFORMATION
- Instruct patients to take Metoclopramide Hydrochloride Orally Disintegrating Tablets at least 30 minutes before eating and at bedtime.
- A patient Medication Guide is available for Metoclopramide Hydrochloride Orally Disintegrating Tablets and printed at the end of the prescribing information. Instruct patients, families, and caregivers to read the Medication Guide and assist them in understanding its contents.
- Inform patients or their caregivers of serious potential issues associated with metoclopramide use such as tardive dyskinesia, extrapyramidal symptoms, and neuroleptic malignant syndrome. Advise patients to inform their physician if symptoms associated with these disorders occur during or after treatment with Metoclopramide Hydrochloride Orally Disintegrating Tablets.
- Inform patients that Metoclopramide Hydrochloride Orally Disintegrating Tablets may cause drowsiness, dizziness, or otherwise impair mental alertness or physical abilities required for the performance of hazardous tasks such as operating machinery or driving a motor vehicle. Sedation may be more pronounced in the elderly.
- Inform patients that the most common adverse reactions in patients treated with Metoclopramide Hydrochloride Orally Disintegrating Tablets or other metoclopramide-containing products are headache, nausea, vomiting, tiredness, sleepiness, dizziness, and restlessness.
Novel Laboratories, Inc
Somerset, NJ 08873
USA
PI5800000102
Rev. 05/2017
-
SPL MEDGUIDE
Metoclopramide Hydrochloride Orally Disintegrating Tablets
Phenylketonurics:
Phenylalanine is a component of aspartame. Each 5 mg and 10 mg Metoclopramide Hydrochloride Orally Disintegrating Tablets contains 4.7 mg of phenylalanine.
Read the Medication Guide that comes with Metoclopramide Hydrochloride Orally Disintegrating Tablets before you take it and each time you get a refill. There may be new information. If you take another product that contains metoclopramide (such as REGLAN tablets, REGLAN ODT, REGLAN injection or metoclopramide oral solution), you should read the Medication Guide that comes with that product. Some of the information may be different. This Medication Guide does not take the place of talking with your doctor about your medical condition or your treatment.
What is the most important information I should know about Metoclopramide Hydrochloride Orally Disintegrating Tablets?
Metoclopramide Hydrochloride Orally Disintegrating Tablets can cause serious side effects, including:
Tardive Dyskinesia (abnormal muscle movements) These movements happen mostly in the face muscles. You cannot control these movements. They may not go away even after stopping Metoclopramide Hydrochloride Orally Disintegrating Tablets. There is no treatment for tardive dyskinesia, but symptoms may lessen or go away over time after you stop taking Metoclopramide Hydrochloride Orally Disintegrating Tablets.
Your chances for getting tardive dyskinesia go up:
- the longer you take Metoclopramide Hydrochloride Orally Disintegrating Tablets and the more Metoclopramide Hydrochloride Orally Disintegrating Tablets you take. You should not take Metoclopramide Hydrochloride Orally Disintegrating Tablets for more than 12 weeks.
- if you are older, especially if you are an older woman
- if you have diabetes
It is not possible for your doctor to know if you will get tardive dyskinesia if you take Metoclopramide Hydrochloride Orally Disintegrating Tablets.
Call your doctor right away if you have movements you can not stop or control, such as:
- lip smacking, chewing, or puckering of your lips
- frowning or scowling
- sticking out your tongue
- blinking and moving your eyes
- shaking of your arms and legs
See the section "What are the possible side effects of Metoclopramide Hydrochloride Orally Disintegrating Tablets?" for more information about side effects.
What is Metoclopramide Hydrochloride Orally Disintegrating Tablets?
Metoclopramide Hydrochloride Orally Disintegrating Tablets is a prescription medicine used in adults:
- for 4 to 12 weeks to relieve heartburn symptoms of gastroesophageal reflux disease (GERD) when certain other treatments do not work.
- to relieve the symptoms of slow stomach emptying in people with diabetes.
It is not known if Metoclopramide Hydrochloride Orally Disintegrating Tablets is safe or works in children.
Who should not take Metoclopramide Hydrochloride Orally Disintegrating Tablets?
Do not take Metoclopramide Hydrochloride Orally Disintegrating Tablets if you:
- have stomach or intestine problems that could get worse with Metoclopramide Hydrochloride Orally Disintegrating Tablets, such as bleeding, blockage or a tear in your stomach or bowel wall
- have an adrenal tumor called pheochromocytoma
- are allergic to metoclopramide or any of the ingredients in Metoclopramide Hydrochloride Orally Disintegrating Tablets. See the end of this Medication Guide for a list of ingredients in Metoclopramide Hydrochloride Orally Disintegrating Tablets.
- take medicines that can cause uncontrolled movements, such as medicines for mental illness.
- have seizures
What should I tell my doctor before taking Metoclopramide Hydrochloride Orally Disintegrating Tablets?
Before you take Metoclopramide Hydrochloride Orally Disintegrating Tablets, tell your doctor if you:
- have kidney or liver disease
- have depression or mental illness
- have high blood pressure
- have heart failure or heart rhythm problems
- have diabetes. Your dose of insulin may need to be changed.
- have Parkinson's disease
- have any other medical conditions
- drink alcohol
- are pregnant or plan to become pregnant. It is not known if Metoclopramide Hydrochloride Orally Disintegrating Tablets will harm your unborn baby.
- are breast-feeding or plan to breast-feed. Metoclopramide Hydrochloride Orally Disintegrating Tablets can pass into your milk and may harm your baby. You and your doctor should decide if you will take Metoclopramide Hydrochloride Orally Disintegrating Tablets or breast-feed. You should not do both.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Metoclopramide Hydrochloride Orally Disintegrating Tablets and some medicines can affect each other and may not work as well, or cause possible side effects. Do not start any new medicine while taking Metoclopramide Hydrochloride Orally Disintegrating Tablets until you talk with your doctor.
Especially tell your doctor if you take:
- another medicine that contains metoclopramide, such as REGLAN tablets, REGLAN ODT, or metoclopramide oral syrup
- a blood pressure medicine
- a medicine for depression, especially a monoamine oxidase inhibitor (MAOI)
- an anti-psychotic medicine
- insulin
- medicines that can make you sleepy, such as anti-anxiety medicines, sleep medicines, and narcotics.
Ask your doctor or pharmacist if you are not sure if your medication is listed above. Know the medicines you take. Keep a list of your medicines to show your doctor and pharmacist when you get new medicine.
How should I take Metoclopramide Hydrochloride Orally Disintegrating Tablets?
- Metoclopramide Hydrochloride Orally Disintegrating Tablets comes as a tablet that melts in your mouth.
- Take Metoclopramide Hydrochloride Orally Disintegrating Tablets exactly as prescribed by your doctor. Do not change your dose unless your doctor tells you to.
- You should not take Metoclopramide Hydrochloride Orally Disintegrating Tablets for more than 12 weeks.
- Take Metoclopramide Hydrochloride Orally Disintegrating Tablets at least 30 minutes before eating and at bedtime.
To take Metoclopramide Hydrochloride Orally Disintegrating Tablets:
- Leave the tablet in the sealed blister Metoclopramide Hydrochloride Orally Disintegrating Tablets pack until you are ready to take it.
- Use dry hands to open a blister and take out a tablet. If the tablet breaks or crumbles throw it away and take a new tablet out of the blister pack.
- Put the tablet on your tongue right away. Let it melt and then swallow. You do not need water to take Metoclopramide Hydrochloride Orally Disintegrating Tablets.
If you take too much Metoclopramide Hydrochloride Orally Disintegrating Tablets, call your doctor or Poison Control Center.
What should I avoid while taking Metoclopramide Hydrochloride Orally Disintegrating Tablets?
- Do not drink alcohol while taking Metoclopramide Hydrochloride Orally Disintegrating Tablets. Alcohol may make some side effects of Metoclopramide Hydrochloride Orally Disintegrating Tablets worse, such as feeling sleepy.
- Do not drive, work with machines, or do dangerous tasks until you know how Metoclopramide Hydrochloride Orally Disintegrating Tablets affects you. Metoclopramide Hydrochloride Orally Disintegrating Tablets may cause sleepiness.
What are the possible side effects of Metoclopramide Hydrochloride Orally Disintegrating Tablets?
Metoclopramide Hydrochloride Orally Disintegrating Tablets can cause serious side effects, including:
- Tardivedyskinesia (abnormal muscle movements) See "What is the most important information I should know about Metoclopramide Hydrochloride Orally Disintegrating Tablets?"
- Uncontrolled spasms of your face and neck muscles, or muscles of your body, arms, and legs (dystonia). These muscle spasms can cause abnormal movements and body positions. These spasms usually start within the first 2 days of treatment. These spasms happen more often in children and adults younger than 30.
- Depression, thoughts about suicide, and suicide. Some people who take Metoclopramide Hydrochloride Orally Disintegrating Tablets may become depressed. You may have thoughts about hurting or killing yourself. Some people who have taken metoclopramide products have ended their own lives (suicide).
- Neuroleptic Malignant Syndrome (NMS). NMS is a rare but very serious condition that can happen with Metoclopramide Hydrochloride Orally Disintegrating Tablets. NMS can cause death and must be treated in a hospital. Symptoms of NMS include: high fever, stiff muscles, problems thinking, very fast or uneven heartbeat, and increased sweating.
- Parkinsonism. Symptoms include slight shaking, body stiffness, trouble moving or keeping your balance. If you have Parkinson's Disease, your symptoms may become worse while you are taking Metoclopramide Hydrochloride Orally Disintegrating Tablets.
- High blood pressure. Metoclopramide Hydrochloride Orally Disintegrating Tablets can cause your blood pressure to increase.
- Too much body water. People who have certain liver problems or heart failure and take Metoclopramide Hydrochloride Orally Disintegrating Tablets may hold too much water in their body (fluid retention). Tell your doctor right away if you have sudden weight gain, or swelling of your hands, legs, or feet.
Call your doctor and get medical help right away if you:
- feel depressed or have thoughts about hurting or killing yourself
- have high fever, stiff muscles, problems thinking, very fast or uneven heartbeat, and increased sweating
- have muscle movements you cannot stop or control
- have muscle movements that are new or unusual
The most common side effects of Metoclopramide Hydrochloride Orally Disintegrating Tablets are:
- headache
- nausea
- vomiting
- tiredness
- sleepiness
You may have more side effects the longer you take Metoclopramide Hydrochloride Orally Disintegrating Tablets and the more Metoclopramide Hydrochloride Orally Disintegrating Tablets you take.
You may still have side effects after you stop Metoclopramide Hydrochloride Orally Disintegrating Tablets. You may have symptoms from stopping (withdrawal) Metoclopramide Hydrochloride Orally Disintegrating Tablets such as headaches, and feeling dizzy or nervous.
Tell your doctor about any side effects that bother you or do not go away. These are not all the possible side effects of Metoclopramide Hydrochloride Orally Disintegrating Tablets.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1–800–FDA-1088.
How do I store Metoclopramide Hydrochloride Orally Disintegrating Tablets?
- Store Metoclopramide Hydrochloride Orally Disintegrating Tablets at room temperature, between 68°F to 77°F (20°C to 25°C).
- Keep Metoclopramide Hydrochloride Orally Disintegrating Tablets away from moisture.
- Throw away any Metoclopramide Hydrochloride Orally Disintegrating Tablets that is not used.
Keep Metoclopramide Hydrochloride Orally Disintegrating Tablets and all medicines away from children.
General information about Metoclopramide Hydrochloride Orally Disintegrating Tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Metoclopramide Hydrochloride Orally Disintegrating Tablets for a condition for which it was not prescribed. Do not give Metoclopramide Hydrochloride Orally Disintegrating Tablets to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about Metoclopramide Hydrochloride Orally Disintegrating Tablets. If you would like more information about Metoclopramide Hydrochloride Orally Disintegrating Tablets, talk with your doctor. You can ask your doctor or pharmacist for information about Metoclopramide Hydrochloride Orally Disintegrating Tablets that is written for health professionals.
What are the ingredients in Metoclopramide Hydrochloride Orally Disintegrating Tablets?
Active ingredients: metoclopramide hydrochloride
Inactive ingredients: phosphoric acid, mannitol and starch, microcrystalline cellulose, colloidal silicon dioxide, amino methacrylate copolymer, butylated hydroxyanisole, butylated hydroxytoluene, crospovidone, aspartame, N-C mint flavor, magnesium stearate.
Novel Laboratories, Inc
Somerset, NJ 08873
USA
PI5800000102
Rev 05/2017
This Medication Guide has been approved by the U.S. Food and Drug Administration.
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 40032-580-30
Blister Pack
Metoclopramide Hydrochloride Orally Disintegrating Tablets
10 mg
NDC: 40032-580-31
Carton
Metoclopramide Hydrochloride Orally Disintegrating Tablets
10 mg
Front Panel
NDC: 40032-581-30
Blister Pack
Metoclopramide Hydrochloride Orally Disintegrating Tablets
5 mg
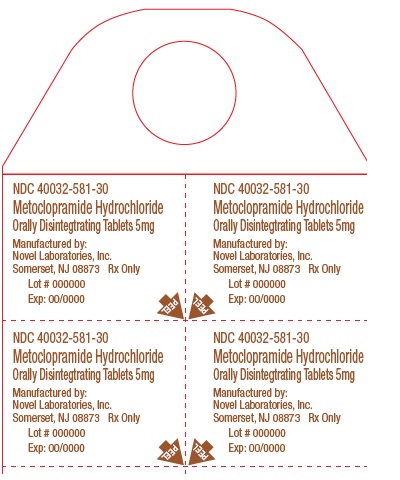
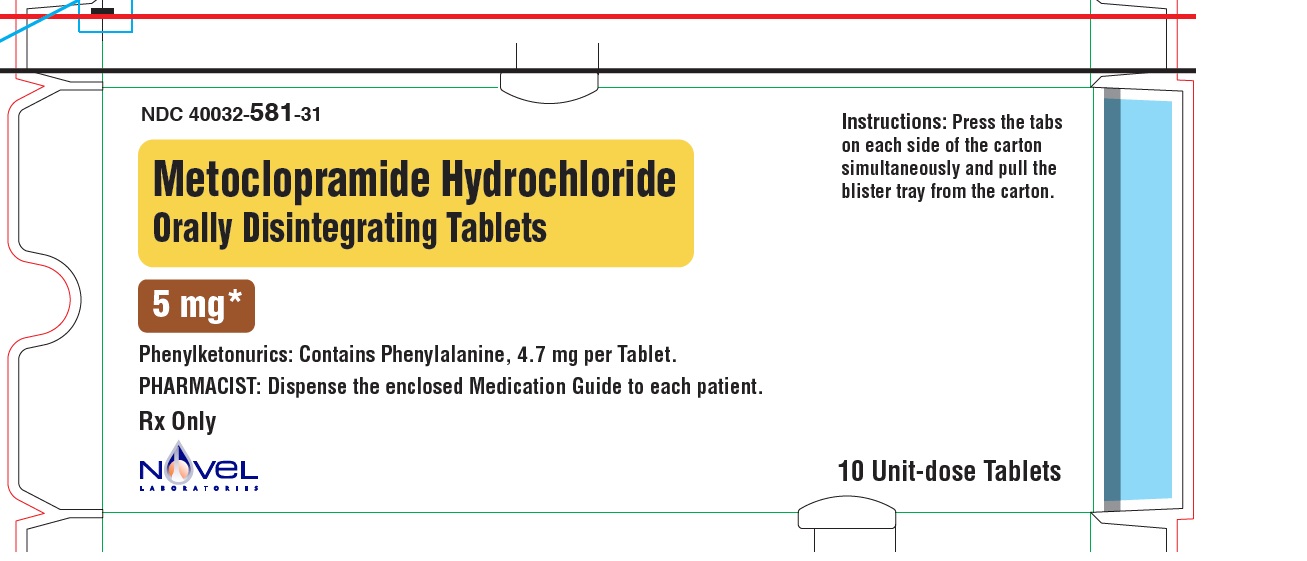
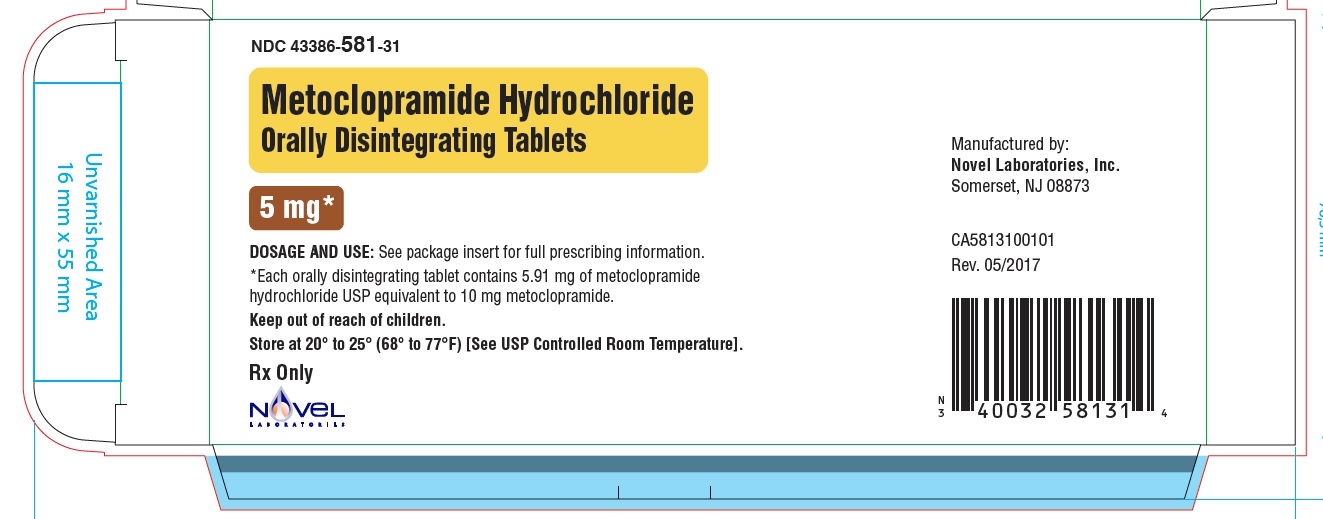
NDC: 40032-581-31
Carton
Metoclopramide Hydrochloride Orally Disintegrating Tablets
5 mg
Front Panel
-
INGREDIENTS AND APPEARANCE
METOCLOPRAMIDE HYDROCHLORIDE
metoclopramide hydrochloride tablet, orally disintegratingProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 40032-580 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METOCLOPRAMIDE HYDROCHLORIDE (UNII: W1792A2RVD) (METOCLOPRAMIDE - UNII:L4YEB44I46) METOCLOPRAMIDE 10 mg Inactive Ingredients Ingredient Name Strength PHOSPHORIC ACID (UNII: E4GA8884NN) MANNITOL (UNII: 3OWL53L36A) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CROSPOVIDONE (UNII: 2S7830E561) ASPARTAME (UNII: Z0H242BBR1) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) DIMETHYLAMINOETHYL METHACRYLATE - BUTYL METHACRYLATE - METHYL METHACRYLATE COPOLYMER (UNII: 905HNO1SIH) Product Characteristics Color WHITE (off-white) Score no score Shape ROUND Size 12mm Flavor Imprint Code N;580 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 40032-580-31 10 in 1 CARTON 08/15/2014 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202191 08/15/2014 METOCLOPRAMIDE HYDROCHLORIDE
metoclopramide hydrochloride tablet, orally disintegratingProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 40032-581 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METOCLOPRAMIDE HYDROCHLORIDE (UNII: W1792A2RVD) (METOCLOPRAMIDE - UNII:L4YEB44I46) METOCLOPRAMIDE 5 mg Inactive Ingredients Ingredient Name Strength PHOSPHORIC ACID (UNII: E4GA8884NN) MANNITOL (UNII: 3OWL53L36A) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CROSPOVIDONE (UNII: 2S7830E561) ASPARTAME (UNII: Z0H242BBR1) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) DIMETHYLAMINOETHYL METHACRYLATE - BUTYL METHACRYLATE - METHYL METHACRYLATE COPOLYMER (UNII: 905HNO1SIH) Product Characteristics Color WHITE (off-white) Score no score Shape ROUND Size 12mm Flavor Imprint Code N;581 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 40032-581-31 10 in 1 CARTON 08/15/2014 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA202191 08/15/2014 Labeler - Novel Laboratories, Inc. (793518643) Registrant - Novel Laboratories, Inc. (793518643) Establishment Name Address ID/FEI Business Operations Novel Laboratories, Inc. 793518643 ANALYSIS(40032-580, 40032-581) , MANUFACTURE(40032-580, 40032-581) , PACK(40032-580, 40032-581)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.