MOTION SICKNESS- dimenhydrinate tablet
Motion Sickness by
Drug Labeling and Warnings
Motion Sickness by is a Otc medication manufactured, distributed, or labeled by Target Corporation, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
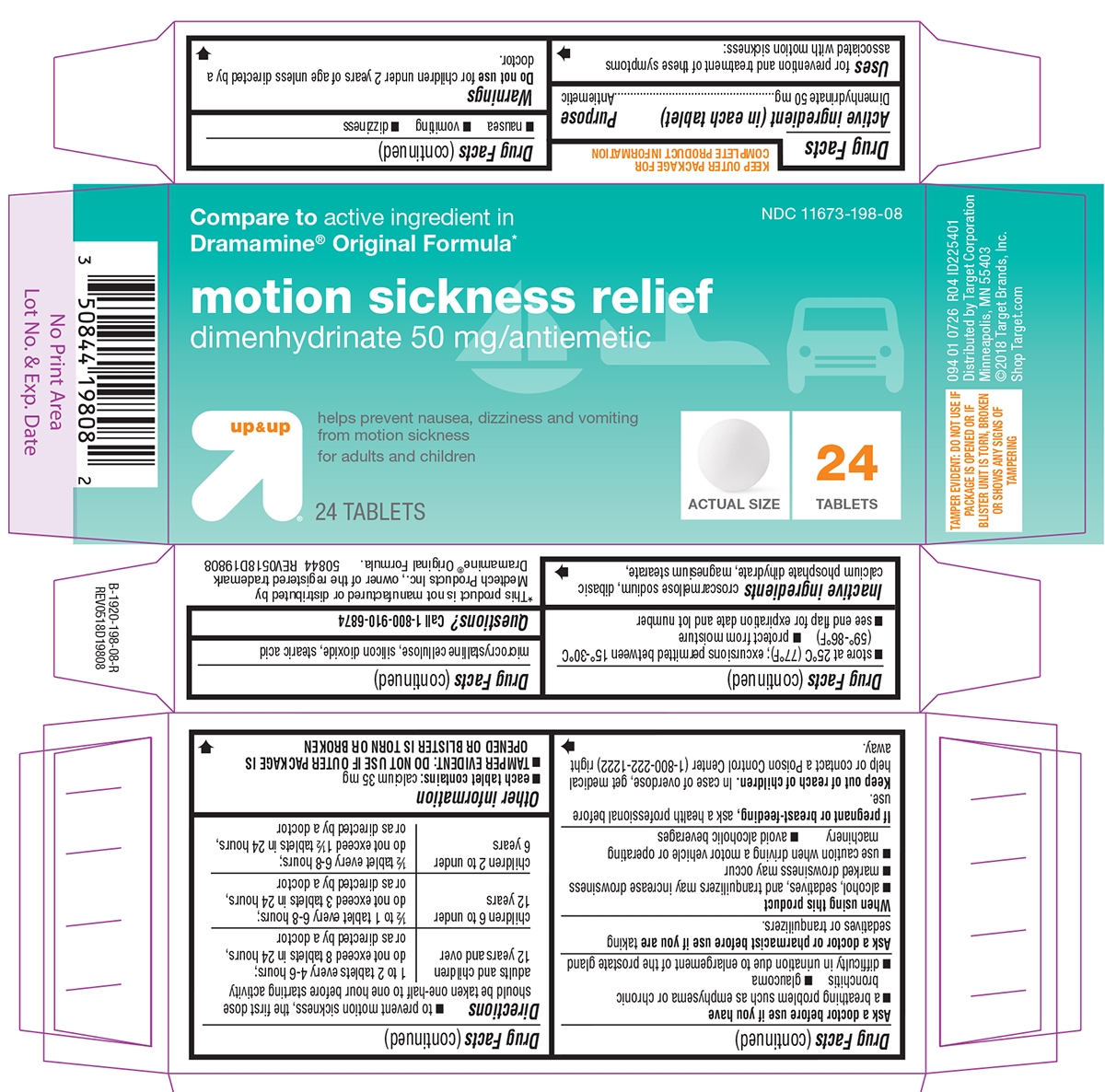
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
-
Directions
- to prevent motion sickness, the first dose should be taken one-half to one hour before starting activity
adults and children 12 years and over 1 to 2 tablets every 4-6 hours; do not exceed 8 tablets in 24 hours, or as directed by a doctor children 6 to under 12 years ½ to 1 tablet every 6-8 hours; do not exceed 3 tablets in 24 hours, or as directed by a doctor children 2 to under 6 years ½ tablet every 6-8 hours; do not exceed 1½ tablets in 24 hours, or as directed by a doctor
- to prevent motion sickness, the first dose should be taken one-half to one hour before starting activity
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
NDC: 11673-198-08
Compare to active ingredient in
Dramamine® Original Formula*motion sickness relief
dimenhydrinate 50 mg/antiemeticup & up
helps prevent nausea, dizziness and vomiting
from motion sicknessfor adults and children
24 TABLETS
ACTUAL SIZE
094 01 0726 RO4 ID225401
Distributed by Target Corporation
Minneapolis, MN 55403
©2018 Target Brands, Inc.
Shop Target.com*This product is not manufactured or distributed by Medtech Products Inc., owner of the registered trademark Dramamine® Original Formula.
50844 REV0518D19808TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
Target 44-198
-
INGREDIENTS AND APPEARANCE
MOTION SICKNESS
dimenhydrinate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 11673-198 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMENHYDRINATE (UNII: JB937PER5C) (DIPHENHYDRAMINE - UNII:8GTS82S83M, CHLORTHEOPHYLLINE - UNII:GE2UA340FM) DIMENHYDRINATE 50 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STEARIC ACID (UNII: 4ELV7Z65AP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) Product Characteristics Color WHITE Score 2 pieces Shape ROUND Size 9mm Flavor Imprint Code 44;198 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11673-198-08 4 in 1 CARTON 12/01/1992 1 6 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 11673-198-02 2 in 1 CARTON 12/01/1992 2 6 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part336 12/01/1992 Labeler - Target Corporation (006961700) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 PACK(11673-198) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 MANUFACTURE(11673-198) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 PACK(11673-198) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 PACK(11673-198) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 PACK(11673-198)
Trademark Results [Motion Sickness]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MOTION SICKNESS 98591174 not registered Live/Pending |
Ronald Joseph Tanksley 2024-06-07 |
 MOTION SICKNESS 87089947 5137431 Live/Registered |
Motion Sickness LLC 2016-06-30 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.