ZO SKIN HEALTH LEVEL II ANTI-AGING PROGRAM WITH GROWTH FACTOR SERUM PLUS- titanium dioxide and zinc oxide kit
ZO SKIN HEALTH by
Drug Labeling and Warnings
ZO SKIN HEALTH by is a Otc medication manufactured, distributed, or labeled by ZO Skin Health, Inc., Lifetech Resources, PakLab, Inc., Product Quest, Owen Biosciences. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure
- Use a water resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours
- Children under 6 months: Ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- - Limit time in the sun, especially from 10 a.m.- 2 p.m.
- - Wear long-sleeved shirts, pants, hats and sunglasses.
- Apply to all skin exposed to the sun
- Other information
-
Inactive ingredients
Cyclopentasiloxane, Cyclotetrasiloxane, Dimethicone/Vinyl Dimethicone Crosspolymer, Methyl Methacrylate/Glycol Dimethacrylate Crosspolymer, Dimethicone Crosspolymer, Melanin, Tribehenin, Calcium Behenate, Sorbitan Isostearate, Dimethiconol, Alumina, Laureth-23, Methicone, PEG-10 Dimethicone, PPG-2 Myristyl Ether Propionate, Propylparaben, Methylparaben, Myristoyl Pentapeptide-8, Iron Oxide (CI 77492).
- SPL UNCLASSIFIED SECTION
-
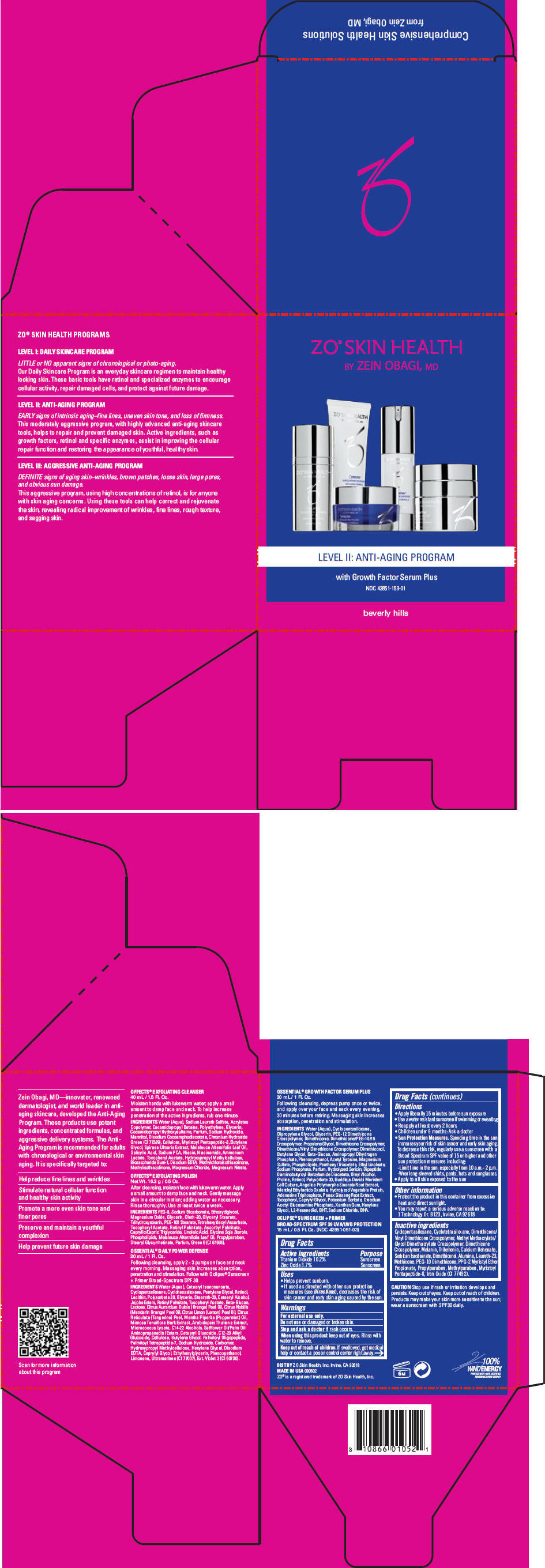
PRINCIPAL DISPLAY PANEL - Kit Carton
ZO® SKIN HEALTH
BY ZEIN OBAGI, MDLEVEL II: ANTI-AGING PROGRAM
with Growth Factor Serum Plus
NDC: 42851-153-01
beverly hills
-
INGREDIENTS AND APPEARANCE
ZO SKIN HEALTH LEVEL II ANTI-AGING PROGRAM WITH GROWTH FACTOR SERUM PLUS
titanium dioxide and zinc oxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 42851-153 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42851-153-01 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 40 mL Part 2 1 JAR 16.2 g Part 3 1 BOTTLE, PUMP 30 mL Part 4 1 BOTTLE, PUMP 30 mL Part 5 1 BOTTLE, PUMP 15 mL Part 1 of 5 OFFECTS EXFOLIATING CLEANSER NORMAL TO DRY SKIN ANTI-AGING FORMULA
cleansing (cold creams, cleansing lotions, liquids, and pads) lotionProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR SODIUM LAURETH-3 SULFATE (UNII: BPV390UAP0) INGR COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) INGR HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR COCAMIDOPROPYL HYDROXYSULTAINE (UNII: 62V75NI93W) INGR SODIUM HYDROXIDE (UNII: 55X04QC32I) INGR MANNITOL (UNII: 3OWL53L36A) INGR Disodium Cocoamphodiacetate (UNII: 18L9G3U51M) INGR CHROMIUM HYDROXIDE GREEN (UNII: RV8FT8XF5R) INGR POWDERED CELLULOSE (UNII: SMD1X3XO9M) INGR Butylene Glycol (UNII: 3XUS85K0RA) INGR FILIPENDULA ULMARIA ROOT (UNII: 997724QNDS) INGR TEA TREE OIL (UNII: VIF565UC2G) INGR Salicylic Acid (UNII: O414PZ4LPZ) INGR SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) INGR NIACIN (UNII: 2679MF687A) INGR NIACINAMIDE (UNII: 25X51I8RD4) INGR AMMONIUM LACTATE (UNII: 67M901L9NQ) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR HYPROMELLOSES (UNII: 3NXW29V3WO) INGR BIOSACCHARIDE GUM-1 (UNII: BB4PU4V09H) INGR EDETATE DISODIUM (UNII: 7FLD91C86K) INGR METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) INGR METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) INGR MAGNESIUM CHLORIDE (UNII: 02F3473H9O) INGR MAGNESIUM NITRATE (UNII: 77CBG3UN78) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 40 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 01/11/2012 Part 2 of 5 OFFECTS EXFOLIATING POLISH ANTI-AGING FORMULA
cleansing (cold creams, cleansing lotions, liquids, and pads) creamProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) INGR SODIUM BICARBONATE (UNII: 8MDF5V39QO) INGR DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) INGR MAGNESIUM OXIDE (UNII: 3A3U0GI71G) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR Oleth-20 (UNII: YTH167I2AG) INGR GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) INGR TRIHYDROXYSTEARIN (UNII: 06YD7896S3) INGR PEG-100 Stearate (UNII: YD01N1999R) INGR TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) INGR ASCORBYL PALMITATE (UNII: QN83US2B0N) INGR MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) INGR LINOLEIC ACID (UNII: 9KJL21T0QJ) INGR OMEGA-3 FATTY ACIDS (UNII: 71M78END5S) INGR TEA TREE OIL (UNII: VIF565UC2G) INGR PROPYLPARABEN (UNII: Z8IX2SC1OH) INGR STEARYL GLYCYRRHETINATE (UNII: 3YYE6VJS0P) INGR D&C GREEN NO. 6 (UNII: 4QP5U84YF7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 16.2 g in 1 JAR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 01/11/2012 Part 3 of 5 OSSENTIAL DAILY POWER DEFENSE ANTI-AGING FORMULA
cleansing (cold creams, cleansing lotions, liquids, and pads) lotionProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR CETEARYL ISONONANOATE (UNII: P5O01U99NI) INGR CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) INGR CYCLOMETHICONE 6 (UNII: XHK3U310BA) INGR PENTYLENE GLYCOL (UNII: 50C1307PZG) INGR RETINOL (UNII: G2SH0XKK91) INGR EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR STEARETH-20 (UNII: L0Q8IK9E08) INGR CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) INGR JOJOBA BUTTER (UNII: XIA46H803R) INGR VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR CURDLAN (UNII: 6930DL209R) INGR LACTOSE (UNII: J2B2A4N98G) INGR ORANGE OIL (UNII: AKN3KSD11B) INGR MANDARIN OIL (UNII: NJO720F72R) INGR LEMON OIL (UNII: I9GRO824LL) INGR PEPPERMINT OIL (UNII: AV092KU4JH) INGR MIMOSA TENUIFLORA BARK (UNII: 515MQE449I) INGR ARABIDOPSIS THALIANA (UNII: AI3L60HQ81) INGR C14-22 ALCOHOLS (UNII: B1K89384RJ) INGR CETEARYL GLUCOSIDE (UNII: 09FUA47KNA) INGR C12-20 ALKYL GLUCOSIDE (UNII: K67N5Z1RUA) INGR POWDERED CELLULOSE (UNII: SMD1X3XO9M) INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR PALMITOYL OLIGOPEPTIDE (UNII: HO4ZT5S86C) INGR PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) INGR SODIUM HYDROXIDE (UNII: 55X04QC32I) INGR CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) INGR HYPROMELLOSES (UNII: 3NXW29V3WO) INGR HEXYLENE GLYCOL (UNII: KEH0A3F75J) INGR EDETATE DISODIUM (UNII: 7FLD91C86K) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR LIMONENE, (+/-)- (UNII: 9MC3I34447) INGR ULTRAMARINE BLUE (UNII: I39WR998BI) INGR EXT. D&C VIOLET NO. 2 (UNII: G5UX3K0728) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 01/11/2012 Part 4 of 5 OSSENTIAL GROWTH FACTOR SERUM PLUS ANTI-AGING FORMULA
cleansing (cold creams, cleansing lotions, liquids, and pads) gelProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) INGR DIPROPYLENE GLYCOL (UNII: E107L85C40) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR PEG-12 DIMETHICONE/PPG-20 CROSSPOLYMER (UNII: 965K72OQXO) INGR DIMETHICONE (UNII: 92RU3N3Y1O) INGR PROPYLENE GLYCOL (UNII: 6DC9Q167V3) INGR DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) INGR DIMETHICONOL (40 CST) (UNII: 343C7U75XW) INGR BUTYLENE GLYCOL (UNII: 3XUS85K0RA) INGR CURDLAN (UNII: 6930DL209R) INGR AMINOPROPYL DIHYDROGEN PHOSPHATE (UNII: D5J0Q2CM4V) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR N-ACETYL-TYROSINE (UNII: DA8G610ZO5) INGR MAGNESIUM SULFATE (UNII: DE08037SAB) INGR OMEGA-3 FATTY ACIDS (UNII: 71M78END5S) INGR PANTHENOL TRIACETATE, (+)- (UNII: 1206E8961B) INGR ETHYL LINOLEATE (UNII: MJ2YTT4J8M) INGR SODIUM PHOSPHATE (UNII: SE337SVY37) INGR HYDROLYZED SERICIN (ENZYMATIC; 2500 MW) (UNII: 02EMA6F96C) INGR DIPEPTIDE DIAMINOBUTYROYL BENZYLAMIDE DIACETATE (UNII: 38H206R00R) INGR OLEYL ALCOHOL (UNII: 172F2WN8DV) INGR PROLINE (UNII: 9DLQ4CIU6V) INGR Retinol (UNII: G2SH0XKK91) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR ANGELICA SINENSIS ROOT (UNII: B66F4574UG) INGR ADENOSINE TRIPHOSPHATE (UNII: 8L70Q75FXE) INGR ASIAN GINSENG (UNII: CUQ3A77YXI) INGR TOCOPHEROL (UNII: R0ZB2556P8) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR POTASSIUM SORBATE (UNII: 1VPU26JZZ4) INGR XANTHAN GUM (UNII: TTV12P4NEE) INGR HEXYLENE GLYCOL (UNII: KEH0A3F75J) INGR 1,2-HEXANEDIOL (UNII: TR046Y3K1G) INGR BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) INGR Sodium Chloride (UNII: 451W47IQ8X) INGR BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 01/11/2012 Part 5 of 5 OCLIPSE SUNSCREEN PLUS PRIMER BROAD-SPECTRUM SPF 30 UVA/UVB PROTECTION
titanium dioxide and zinc oxide lotionProduct Information Item Code (Source) NDC: 42851-051 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 102 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 37 mg in 1 mL Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 4 (UNII: CZ227117JE) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) TRIBEHENIN (UNII: 8OC9U7TQZ0) CALCIUM BEHENATE (UNII: J5VFA9V6YG) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) DIMETHICONOL (40 CST) (UNII: 343C7U75XW) ALUMINUM OXIDE (UNII: LMI26O6933) LAURETH-23 (UNII: N72LMW566G) METHICONE (20 CST) (UNII: 6777U11MKT) PEG-10 DIMETHICONE (600 CST) (UNII: 8PR7V1SVM0) PPG-2 MYRISTYL ETHER PROPIONATE (UNII: 88R97D8U8A) PROPYLPARABEN (UNII: Z8IX2SC1OH) METHYLPARABEN (UNII: A2I8C7HI9T) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42851-051-01 15 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/11/2012 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/11/2012 Labeler - ZO Skin Health, Inc. (826468527) Establishment Name Address ID/FEI Business Operations Lifetech Resources 622559110 MANUFACTURE(42851-153) Establishment Name Address ID/FEI Business Operations PakLab, Inc. 177711082 MANUFACTURE(42851-153) Establishment Name Address ID/FEI Business Operations Product Quest 927768135 MANUFACTURE(42851-153) Establishment Name Address ID/FEI Business Operations Owen Biosciences 790003045 MANUFACTURE(42851-153)
Trademark Results [ZO SKIN HEALTH]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ZO SKIN HEALTH 98618980 not registered Live/Pending |
Shiji Chuangmei (Xiamen) Brand Management Co., Ltd. 2024-06-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.