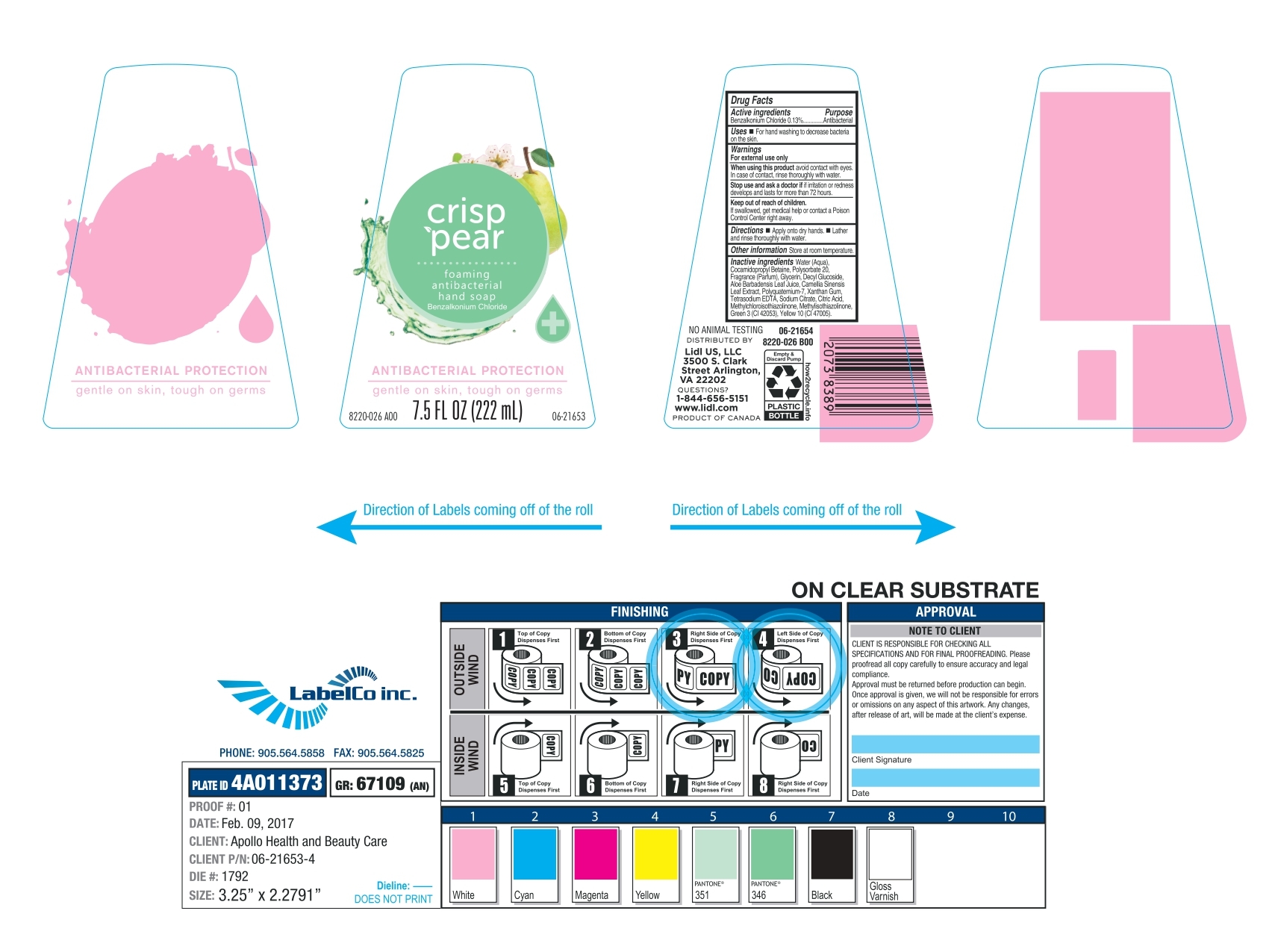
Crisp Pear by Spa Dent Inc / Apollo Health and Beauty Care Drug Facts
Crisp Pear by
Drug Labeling and Warnings
Crisp Pear by is a Otc medication manufactured, distributed, or labeled by Spa Dent Inc, Apollo Health and Beauty Care. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
CRISP PEAR- benzalkonium chloride soap
Spa Dent Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Inactive Ingredients
Water (Aqua), Cocamidopropyl Betaine, Polysorbate 20, Fragrance (Parfum), Glycerin, Decyl Glucoside, Aloe Barbadensis Leaf Juice, Camellia Sinensis Leaf Extract, Polyquatermium-7, Xanthan Gum, Tetrasodium EDTA, Sodium Citrate, Citric Acid, Methylchloroisothiazolinone, Methylisothiazolinone, Green 3 (Cl 42053), Yellow 10 (Cl 47005)
| CRISP PEAR
benzalkonium chloride soap |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Spa Dent Inc (203478896) |
| Registrant - Apollo Health and Beauty Care (201901209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Spa Dent Inc | 203478896 | manufacture(79147-020) | |
Trademark Results [Crisp Pear]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CRISP PEAR 73613782 1495287 Dead/Cancelled |
AMERICAN BEVERAGE MARKETERS, INC. 1986-08-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.