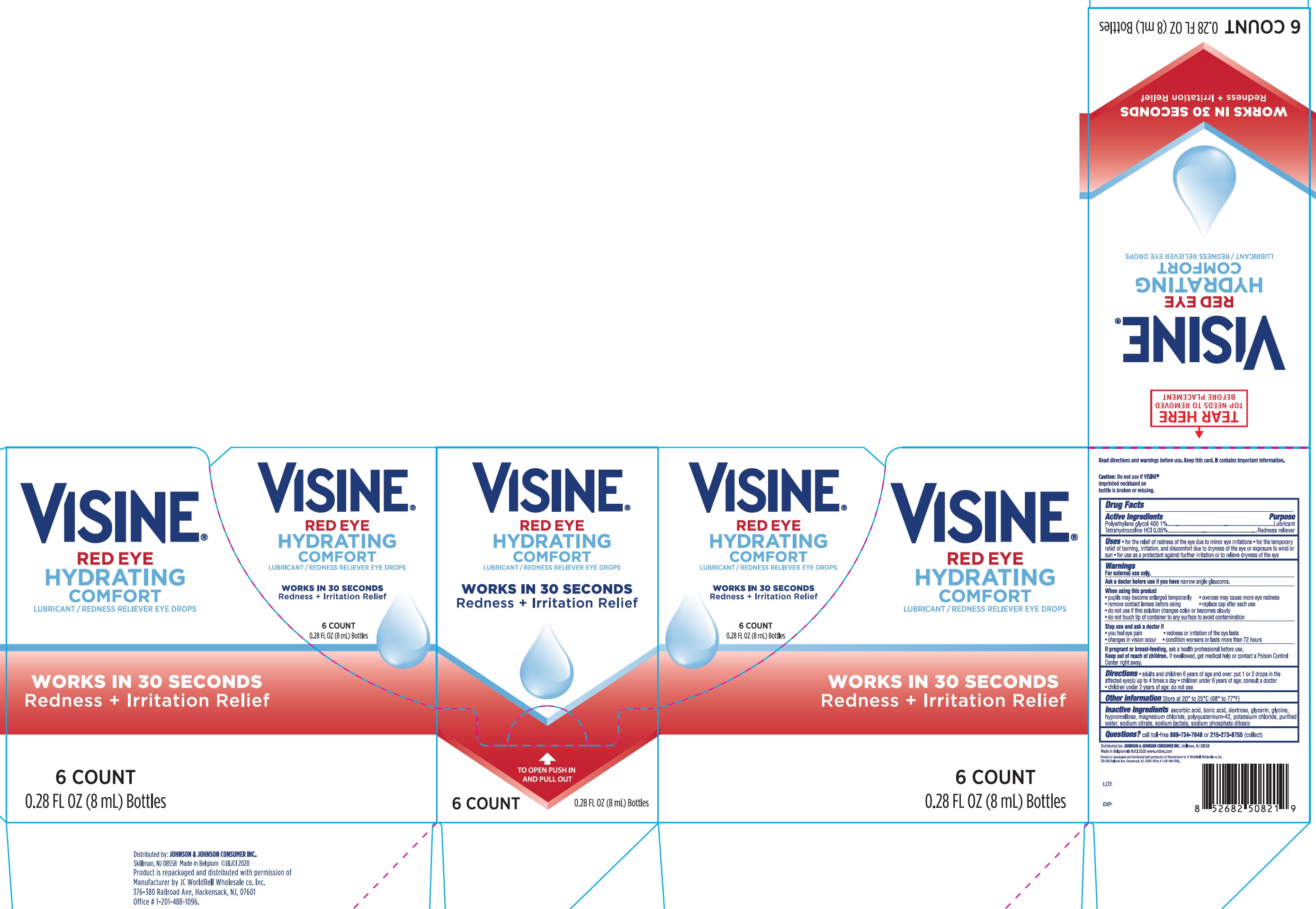
Visine Red Eye Hydrating Comfort
Visine Red Eye Hydrating Comfort by
Drug Labeling and Warnings
Visine Red Eye Hydrating Comfort by is a Otc medication manufactured, distributed, or labeled by Johnson & Johnson Consumer Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
VISINE RED EYE HYDRATING COMFORT- polyethylene glycol 400, tetrahydrozoline hydrochloride liquid
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Visine Red Eye Hydrating Comfort
Uses
- for the relief of redness of the eye due to minor eye irritations
- for the temporary relief of burning, irritation, and discomfort due to dryness of the eye or exposure to wind or sun
- for use as a protectant against further irritation or to relieve dryness of the eye
Warnings
For external use only.
When using this product
- pupils may become enlarged temporarily
- overuse may cause more eye redness
- remove contact lenses before using
- replace cap after each use
- do not use if this solution changes color or becomes cloudy
- do not touch tip of container to any surface to avoid contamination
Directions
- adults and children 6 years of age and over: put 1 or 2 drops in the affected eye(s) up to 4 times a day
- children under 6 years of age: consult a doctor
- children under 2 years of age: do not use
| VISINE RED EYE HYDRATING COMFORT
polyethylene glycol 400, tetrahydrozoline hydrochloride liquid |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc. (002347102) |
Revised: 3/2021
Document Id: bd323c59-5960-c0b7-e053-2a95a90a8ad9
Set id: 402042d7-fd2b-470f-96e1-5fdb7703182a
Version: 2
Effective Time: 20210310