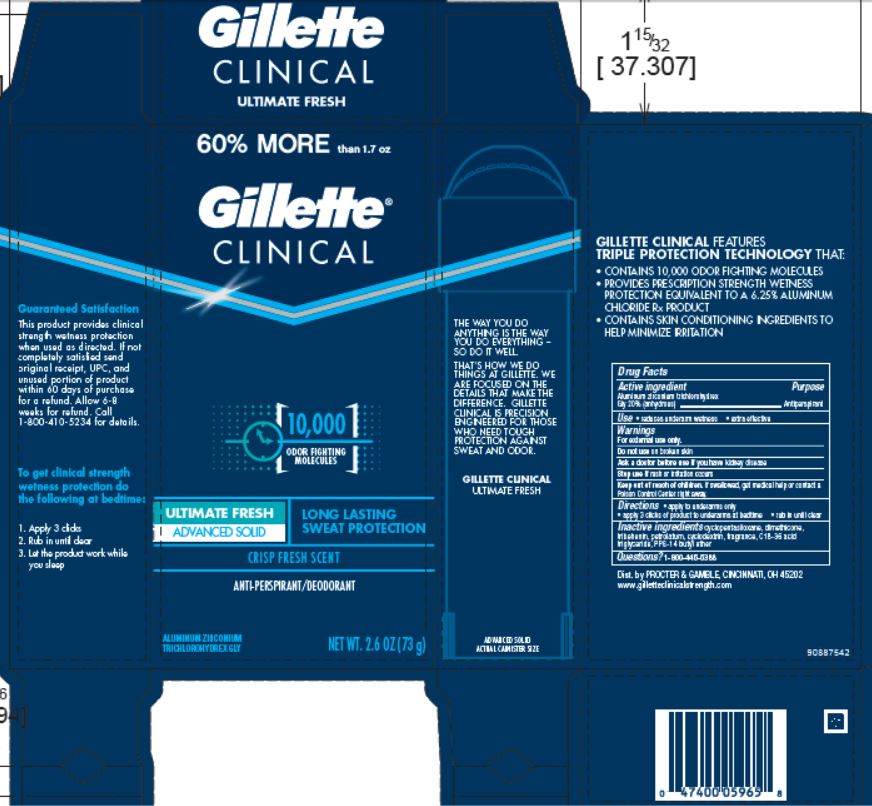
Gillette ® Clinical Advanced Ultimate Fresh
Gillette Clinical Advanced Ultimate Fresh by
Drug Labeling and Warnings
Gillette Clinical Advanced Ultimate Fresh by is a Otc medication manufactured, distributed, or labeled by The Procter & Gamble Manufacturing Company, Procter and Gamble Manufacturing Co., The Gillette Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
GILLETTE CLINICAL ADVANCED ULTIMATE FRESH- aluminum zirconium trichlorohydrex gly cream
The Procter & Gamble Manufacturing Company
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Gillette ® Clinical Advanced Ultimate Fresh
Directions
- apply to underarms only
- apply 3 clicks of product to underarms at bedtime
- rub in until clear
| GILLETTE CLINICAL ADVANCED ULTIMATE FRESH
aluminum zirconium trichlorohydrex gly cream |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - The Procter & Gamble Manufacturing Company (004238200) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Procter and Gamble Manufacturing Co. | 017745779 | manufacture(69423-112) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| The Gillette Company | 047031273 | api manufacture(69423-112) | |
Revised: 12/2017
Document Id: 60642938-5fbd-1daa-e053-2a91aa0a0cfb
Set id: 403ef5c7-b798-029f-e054-00144ff8d46c
Version: 2
Effective Time: 20171215
The Procter & Gamble Manufacturing Company