PHENELZINE SULFATE tablet
Phenelzine Sulfate by
Drug Labeling and Warnings
Phenelzine Sulfate by is a Prescription medication manufactured, distributed, or labeled by Lupin Pharmaceuticals,Inc., Novel Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
BOXED WARNING
(What is this?)
Suicidality and Antidepressant Drugs
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of Phenelzine Sulfate Tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Phenelzine Sulfate Tablets is not approved for use in pediatric patients. (See Warnings: Clinical Worsening and Suicide Risk, Precautions: Information for Patients, and Precautions: Pediatric Use)
-
DESCRIPTION
Phenelzine Sulfate Tablets, USP (phenelzine sulfate) is a potent inhibitor of monoamine oxidase (MAO). Phenelzine sulfate is a hydrazine derivative. It has a molecular weight of 234.27 and is chemically described as C8H12N2 H2SO4. Its chemical structure is shown below:
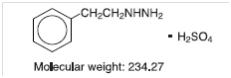
Each Phenelzine Sulfate Tablets film-coated for oral administration contains phenelzine sulfate equivalent to 15 mg of phenelzine base and the following inactive ingredients: mannitol, USP; colloidal silicon dioxide, NF; povidone, USP; edetate disodium, USP; magnesium stearate, NF; purified water, USP; polyvinyl alcohol part hydrolyzed USP, polyethylene glycol-3350 NF, FD&C yellow # 6, talc USP and titanium dioxide USP.
-
CLINICAL PHARMACOLOGY
Monoamine oxidase is a complex enzyme system, widely distributed throughout the body. Drugs that inhibit monoamine oxidase in the laboratory are associated with a number of clinical effects. Thus, it is unknown whether MAO inhibition per se, other pharmacologic actions, or an interaction of both is responsible for the clinical effects observed. Therefore, the physician should become familiar with all the effects produced by drugs of this class.
Pharmacokinetics
Following a single 30 mg dose of Phenelzine Sulfate Tablets (2 × 15 mg tablets), a mean peak plasma concentration (Cmax) of 19.8 ng/mL occurred at a time (Tmax) of 43 minutes postdose.
Metabolism
Phenelzine Sulfate Tablets is extensively metabolized, primarily by oxidation via monoamine oxidase. After oral administration of 13C6-phenelzine, 73% of the administered dose was recovered in urine as phenylacetic acid and parahydroxyphenylacetic acid within 96 hours. Acetylation to N2-acetylphenelzine is a minor pathway.
Elimination
The mean elimination half-life after a single 30 mg dose is 11.6 hours. Multiple dose pharmacokinetics have not been studied in man.
-
INDICATIONS AND USAGE
Phenelzine Sulfate Tablets, USP has been found to be effective in depressed patients clinically characterized as "atypical," "nonendogenous," or "neurotic." These patients often have mixed anxiety and depression and phobic or hypochondriacal features. There is less conclusive evidence of its usefulness with severely depressed patients with endogenous features.
Phenelzine Sulfate Tablets should rarely be the first antidepressant drug used. Rather, it is more suitable for use with patients who have failed to respond to the drugs more commonly used for these conditions.
-
CONTRAINDICATIONS
Phenelzine Sulfate Tablets should not be used in patients who are hypersensitive to the drug or its ingredients, with pheochromocytoma, congestive heart failure, severe renal impairment or renal disease, a history of liver disease, or abnormal liver function tests.
The potentiation of sympathomimetic substances and related compounds by MAO inhibitors may result in hypertensive crises (see WARNINGS). Therefore, patients being treated with Phenelzine Sulfate Tablets should not take sympathomimetic drugs (including amphetamines, cocaine, methylphenidate, dopamine, epinephrine, and norepinephrine) or related compounds (including methyldopa, L-dopa, L-tryptophan, L-tyrosine, and phenylalanine). Hypertensive crises during Phenelzine Sulfate Tablets therapy may also be caused by the ingestion of foods with a high concentration of tyramine or dopamine. Therefore, patients being treated with Phenelzine Sulfate Tablets should avoid high protein food that has undergone protein breakdown by aging, fermentation, pickling, smoking, or bacterial contamination. Patients should also avoid cheeses (especially aged varieties), pickled herring, beer, wine, liver, yeast extract (including brewer's yeast in large quantities), dry sausage (including Genoa salami, hard salami, pepperoni, and Lebanon bologna), pods of broad beans (fava beans), and yogurt. Excessive amounts of caffeine and chocolate may also cause hypertensive reactions.
Phenelzine Sulfate Tablets should not be used in combination with dextromethorphan or with CNS depressants such as alcohol and certain narcotics. Excitation, seizures, delirium, hyperpyrexia, circulatory collapse, coma, and death have been reported in patients receiving MAOI therapy who have been given a single dose of meperidine. Phenelzine Sulfate Tablets should not be administered together with or in rapid succession to other MAO inhibitors because HYPERTENSIVE CRISES and convulsive seizures, fever, marked sweating, excitation, delirium, tremor, coma, and circulatory collapse may occur.
Concomitant use with meperidine is contraindicated (see WARNINGS).
A List of MAO Inhibitors by Generic Name Follows:
pargyline hydrochloride
pargyline hydrochloride and methylclothiazide
furazolidone
isocarboxazid
procarbazine
tranylcypromine
Phenelzine Sulfate Tablets should also not be used in combination with buspirone HCl, since several cases of elevated blood pressure have been reported in patients taking MAO inhibitors who were then given buspirone HCl. At least 14 days should elapse between the discontinuation of Phenelzine Sulfate Tablets and the institution of another antidepressant or buspirone HCl, or the discontinuation of another MAO inhibitor and the institution of Phenelzine Sulfate Tablets.
There have been reports of serious reactions (including hyperthermia, rigidity, myoclonic movements and death) when serotoninergic drugs (e.g., dexfenfluramine, fluoxetine, fluvoxamine, paroxetine, sertraline, citalopram, venlafaxine) have been combined with an MAO inhibitor. Therefore, the concomitant use of Phenelzine Sulfate Tablets with serotoninergic agents is contraindicated (see PRECAUTIONS Drug Interactions). At least 14 days should elapse between the discontinuation of an MAO inhibitor and the start of a serotonin re-uptake inhibitor or vice-versa, with the exception of fluoxetine. Allow at least five weeks between discontinuation of fluoxetine and initiation of Phenelzine Sulfate Tablets and at least 14 days between discontinuation of Phenelzine Sulfate Tablets and initiation of fluoxetine, or other serotoninergic agents. Before initiating Phenelzine Sulfate Tablets after using other serotoninergic agents, a sufficient amount of time must be allowed for clearance of the serotoninergic agent and its active metabolites.
The combination of MAO inhibitors and tryptophan has been reported to cause behavioral and neurologic syndromes including disorientation, confusion, amnesia, delirium, agitation, hypomanic signs, ataxia, myoclonus, hyperreflexia, shivering, ocular oscillations, and Babinski signs.
The concurrent administration of an MAO inhibitor and bupropion hydrochloride (Wellbutrin®) is contraindicated. At least 14 days should elapse between discontinuation of an MAO inhibitor and initiation of treatment with bupropion hydrochloride.
Patients taking Phenelzine Sulfate Tablets should not undergo elective surgery requiring general anesthesia. Also, they should not be given cocaine or local anesthesia containing sympathomimetic vasoconstrictors. The possible combined hypotensive effects of Phenelzine Sulfate Tablets and spinal anesthesia should be kept in mind. Phenelzine Sulfate Tablets should be discontinued at least 10 days prior to elective surgery.
MAO inhibitors, including Phenelzine Sulfate Tablets, are contraindicated in patients receiving guanethidine.
-
WARNINGS
Clinical Worsening and Suicide Risk
Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short term placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18–24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
The pooled analyses of placebo-controlled trials in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1000 patients treated) are provided in Table 1.
Table 1
Age
Range
Drug-Placebo Difference in Number of Cases of Suicidality per 1000 Patients
Treated
Increases Compared to Placebo
<18
14 additional cases
18–24
5 additional cases
Decreases Compared to Placebo
25–64
1 fewer case
≥65
6 fewer cases
No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient's presenting symptoms.
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers. Prescriptions for Phenelzine Sulfate Tablets should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose.
Screening Patients for Bipolar Disorder
A major depressive episode may be the initial presentation of bipolar disorder. It is generally believed (though not established in controlled trials) that treating such an episode with an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Whether any of the symptoms described above represent such a conversion is unknown. However, prior to initiating treatment with an antidepressant, patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. It should be noted that Phenelzine Sulfate Tablets are not approved for use in treating bipolar depression.
It should be noted that Phenelzine Sulfate Tablets are not approved for use in treating any indications in the pediatric population.
Angle-Closure Glaucoma
The pupillary dilation that occurs following use of many antidepressant drugs including Phenelzine Sulfate Tablets may trigger an angle closure attack in a patient with anatomically narrow angles who does not have a patent iridectomy.
The most serious reactions to Phenelzine Sulfate Tablets involve changes in blood pressure.
Hypertensive Crises
The most important reaction associated with Phenelzine Sulfate Tablets administration is the occurrence of hypertensive crises, which have sometimes been fatal.
These crises are characterized by some or all of the following symptoms: occipital headache which may radiate frontally, palpitation, neck stiffness or soreness, nausea, vomiting, sweating (sometimes with fever and sometimes with cold, clammy skin), dilated pupils, and photophobia. Either tachycardia or bradycardia may be present and can be associated with constricting chest pain.
NOTE: Intracranial bleeding has been reported in association with the increase in blood pressure.
Blood pressure should be observed frequently to detect evidence of any pressor response in all patients receiving Phenelzine Sulfate Tablets. Therapy should be discontinued immediately upon the occurrence of palpitation or frequent headaches during therapy.
Recommended treatment in hypertensive crisis
If a hypertensive crisis occurs, Phenelzine Sulfate Tablets should be discontinued immediately and therapy to lower blood pressure should be instituted immediately. On the basis of present evidence, phentolamine is recommended. (The dosage reported for phentolamine is 5 mg intravenously.) Care should be taken to administer this drug slowly in order to avoid producing an excessive hypotensive effect. Fever should be managed by means of external cooling.
Warning to the Patient
All patients should be warned that the following foods, beverages, and medications must be avoided while taking Phenelzine Sulfate Tablets, and for two weeks after discontinuing use.
Foods and Beverages To Avoid
Meat and Fish
Pickled herring
Liver
Dry sausage (including Genoa salami, hard salami, pepperoni, and Lebanon bologna)
Vegetables
Broad bean pods (fava bean pods)
Sauerkraut
Dairy Products
Cheese (cottage cheese and cream cheese are allowed)
Yogurt
Beverages
Beer and wine
Alcohol-free and reduced-alcohol beer and wine products
Miscellaneous
Yeast extract (including brewer's yeast in large quantities)
Meat extract
Excessive amounts of chocolate and caffeine
Also, any spoiled or improperly refrigerated, handled, or stored protein-rich foods such as meats, fish, and dairy products, including foods that may have undergone protein changes by aging, pickling, fermentation, or smoking to improve flavor should be avoided.
OTC Medications To Avoid
Cold and cough preparations (including those containing dextromethorphan)
Nasal decongestants (tablets, drops, or spray)
Hay-fever medications
Sinus medications
Asthma inhalant medications
Antiappetite medicines
Weight-reducing preparations
"Pep" pills
L-tryptophan containing preparations
Also, certain prescription drugs should be avoided. Therefore, patients under the care of another physician or dentist should inform him/her that they are taking Phenelzine Sulfate Tablets.
Patients should be warned that the use of the above foods, beverages, or medications may cause a reaction characterized by headache and other serious symptoms due to a rise in blood pressure, with the exception of dextromethorphan which may cause reactions similar to those seen with meperidine. Also, there has been a report of an interaction between Phenelzine Sulfate Tablets and dextromethorphan (ingested as a lozenge) causing drowsiness and bizarre behavior.
Patients should be instructed to report promptly the occurrence of headache or other unusual symptoms.
Concomitant Use with Dibenzazepine Derivative Drugs
If the decision is made to administer Phenelzine Sulfate Tablets concurrently with other antidepressant drugs, or within less than 10 days after discontinuation of antidepressant therapy, the patient should be cautioned by the physician regarding the possibility of adverse drug interaction.
A List of Dibenzazepine Derivative Drugs by Generic Name Follows:
nortriptyline hydrochloride
amitriptyline hydrochloride
perphenazine and amitriptyline hydrochloride
clomipramine hydrochloride
desipramine hydrochloride
imipramine hydrochloride
doxepin
carbamazepine
cyclobenzaprine HCl
amoxapine
maprotiline HCl
trimipramine maleate
protriptyline HCl
mirtazapine
Phenelzine Sulfate Tablets should be used with caution in combination with antihypertensive drugs, including thiazide diuretics and β-blockers, since exaggerated hypotensive effects may result.
Use in Pregnancy
The safe use of Phenelzine Sulfate Tablets during pregnancy or lactation has not been established. The potential benefit of this drug, if used during pregnancy, lactation, or in women of childbearing age, should be weighed against the possible hazard to the mother or fetus.
Doses of Phenelzine Sulfate Tablets in pregnant mice well exceeding the maximum recommended human dose have caused a significant decrease in the number of viable offspring per mouse. In addition, the growth of young dogs and rats has been retarded by doses exceeding the maximum human dose.
-
PRECAUTIONS
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with Phenelzine Sulfate Tablets and should counsel them in its appropriate use. A patient Medication Guide about "Antidepressant Medicines, Depression and other Serious Mental Illness, and Suicidal Thoughts or Actions" is available for Phenelzine Sulfate Tablets. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document.
Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking Phenelzine Sulfate Tablets.
Patients should be advised that taking Phenelzine Sulfate Tablets can cause mild pupillary dilation, which in susceptible individuals, can lead to an episode of angle-closure glaucoma. Pre-existing glaucoma is almost always open-angle glaucoma because angle-closure glaucoma, when diagnosed, can be treated definitively with iridectomy. Open-angle glaucoma is not a risk factor for angle closure glaucoma. Patients may wish to be examined to determine whether they are susceptible to angle closure, and have a prophylactic procedure (e.g., iridectomy), if they are susceptible.
Clinical Worsening and Suicide Risk
Patients, their families, and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, mania, other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down. Families and caregivers of patients should be advised to look for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt. Such symptoms should be reported to the patient's prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patient's presenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behavior and indicate a need for very close monitoring and possibly changes in the medication.
Safety and effectiveness in the pediatric population have not been established (see BOX WARNING and WARNINGS—Clinical Worsening and Suicide Risk)
Anyone considering the use of Phenelzine Sulfate Tablets in a child or adolescent must balance the potential risks with the clinical need.
Phenelzine Sulfate Tablets, as with other hydrazine derivatives, has been reported to induce pulmonary and vascular tumors in an uncontrolled lifetime study in mice.
In depressed patients, the possibility of suicide should always be considered and adequate precautions taken. It is recommended that careful observations of patients undergoing Phenelzine Sulfate Tablets treatment be maintained until control of depression is achieved. If necessary, additional measures (ECT, hospitalization, etc) should be instituted.
All patients undergoing treatment with Phenelzine Sulfate Tablets should be closely followed for symptoms of postural hypotension. Hypotensive side effects have occurred in hypertensive as well as normotensive and hypotensive patients. Blood pressure usually returns to pretreatment levels rapidly when the drug is discontinued or the dosage is reduced.
Because the effect of Phenelzine Sulfate Tablets on the convulsive threshold may be variable, adequate precautions should be taken when treating epileptic patients.
Of the more severe side effects that have been reported with any consistency, hypomania has been the most common. This reaction has been largely limited to patients in whom disorders characterized by hyperkinetic symptoms coexist with, but are obscured by, depressive affect; hypomania usually appeared as depression improved. If agitation is present, it may be increased with Phenelzine Sulfate Tablets.
Hypomania and agitation have also been reported at higher than recommended doses or following long-term therapy.
Phenelzine Sulfate Tablets may cause excessive stimulation in schizophrenic patients; in manic-depressive states it may result in a swing from a depressive to a manic phase.
Phenelzine Sulfate Tablets should be used with caution in diabetes mellitus; increased insulin sensitivity may occur. Requirements for insulin or oral hypoglycemics may be decreased.
MAO inhibitors, including Phenelzine Sulfate Tablets, potentiate hexobarbital hypnosis in animals. Therefore, barbiturates should be given at a reduced dose with Phenelzine Sulfate Tablets.
MAO inhibitors inhibit the destruction of serotonin and norepinephrine, which are believed to be released from tissue stores by rauwolfia alkaloids. Accordingly, caution should be exercised when rauwolfia is used concomitantly with an MAO inhibitor, including Phenelzine Sulfate Tablets.
There is conflicting evidence as to whether or not MAO inhibitors affect glucose metabolism or potentiate hypoglycemic agents. This should be kept in mind if Phenelzine Sulfate Tablets is administered to diabetics.
Drug Interactions
In patients receiving nonselective monoamine oxidase (MAO) inhibitors in combination with serotoninergic agents (e.g., dexfenfluramine, fluoxetine, fluvoxamine, paroxetine, sertraline, citalopram, venlafaxine) there have been reports of serious, sometimes fatal, reactions. Because Phenelzine Sulfate Tablets is a monoamine oxidase (MAO) inhibitor, Phenelzine Sulfate Tablets should not be used concomitantly with a serotoninergic agent (See CONTRAINDICATIONS).
Administration of guanethidine to patients receiving an MAO inhibitor can produce moderate to severe hypertension due to release of catecholamines. At least two weeks should elapse between withdrawal of the MAO inhibitor and the initiation of guanethidine. (see CONTRAINDICATIONS)
Geriatric Use
Clinical studies of Phenelzine Sulfate Tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
Phenelzine Sulfate Tablets is a potent inhibitor of monoamine oxidase. Because this enzyme is widely distributed throughout the body, diverse pharmacologic effects can be expected to occur. When they occur, such effects tend to be mild or moderate in severity (see below), often subside as treatment continues, and can be minimized by adjusting dosage; rarely is it necessary to institute counteracting measures or to discontinue Phenelzine Sulfate Tablets.
Common side effects include:
Nervous System —Dizziness, headache, drowsiness, sleep disturbances (including insomnia and hypersomnia), fatigue, weakness, tremors, twitching, myoclonic movements, hyperreflexia.
Gastrointestinal—Constipation, dry mouth, gastrointestinal disturbances, elevated serum transaminases (without accompanying signs and symptoms).
Metabolic —Weight gain.
Cardiovascular —Postural hypotension, edema.
Genitourinary—Sexual disturbances, eg, anorgasmia and ejaculatory disturbances and impotence.
Less common mild to moderate side effects (some of which have been reported in a single patient or by a single physician) include:
Nervous System —Jitteriness, palilalia, euphoria, nystagmus, paresthesias.
Genitourinary—Urinary retention.
Metabolic —Hypernatremia.
Dermatologic —Pruritus, skin rash, sweating.
Special Senses —Blurred vision, angle-closure glaucoma.
Although reported less frequently, and sometimes only once, additional severe side effects include:
Nervous System—Ataxia, shock-like coma, toxic delirium, manic reaction, convulsions, acute anxiety reaction, precipitation of schizophrenia, transient respiratory and cardiovascular depression following ECT.
Gastrointestinal—To date, fatal progressive necrotizing hepatocellular damage has been reported in very few patients. Reversible jaundice.
Hematologic —Leukopenia.
Immunologic—Lupus-like syndrome
Metabolic—Hypermetabolic syndrome (which may include, but is not limited to, hyperpyrexia, tachycardia, tachypnea, muscular rigidity, elevated CK levels, metabolic acidosis, hypoxia, coma and may resemble an overdose).
Respiratory—Edema of the glottis.
General—Fever associated with increased muscle tone.
Withdrawal may be associated with nausea, vomiting, and malaise.
An uncommon withdrawal syndrome following abrupt withdrawal of Phenelzine Sulfate Tablets has been infrequently reported. Signs and symptoms of this syndrome generally commence 24 to 72 hours after drug discontinuation and may range from vivid nightmares with agitation to frank psychosis and convulsions. This syndrome generally responds to reinstitution of low-dose Phenelzine Sulfate Tablets therapy followed by cautious downward titration and discontinuation.
-
DOSAGE AND ADMINISTRATION
The usual starting dose of Phenelzine Sulfate Tablets is one tablet (15 mg) three times a day.
Early phase treatment
Dosage should be increased to at least 60 mg per day at a fairly rapid pace consistent with patient tolerance. It may be necessary to increase dosage up to 90 mg per day to obtain sufficient MAO inhibition. Many patients do not show a clinical response until treatment at 60 mg has been continued for at least 4 weeks.
Maintenance dose
After maximum benefit from Phenelzine Sulfate Tablets is achieved, dosage should be reduced slowly over several weeks. Maintenance dose may be as low as one tablet, 15 mg, a day or every other day, and should be continued for as long as is required.
-
OVERDOSAGE
Note—For management of hypertensive crises see WARNINGS section.
Accidental or intentional overdosage may be more common in patients who are depressed. It should be remembered that multiple drugs and/or alcohol may have been ingested.
Depending on the amount of overdosage with Phenelzine Sulfate Tablets, a varying and mixed clinical picture may develop, including signs and symptoms of central nervous system and cardiovascular stimulation and/or depression. Signs and symptoms may be absent or minimal during the initial 12-hour period following ingestion and may develop slowly thereafter, reaching a maximum in 24–48 hours. Death has been reported following overdosage. Therefore, immediate hospitalization, with continuous patient observation and monitoring throughout this period, is essential.
Signs and symptoms of overdosage may include, alone or in combination, any of the following: drowsiness, dizziness, faintness, irritability, hyperactivity, agitation, severe headache, hallucinations, trismus, opisthotonus, rigidity, convulsions, and coma; rapid and irregular pulse, hypertension, hypotension, and vascular collapse; precordial pain, respiratory depression and failure, hyperpyrexia, diaphoresis, and cool, clammy skin.
Treatment
Intensive symptomatic and supportive treatment may be required. Induction of emesis or gastric lavage with instillation of charcoal slurry may be helpful in early poisoning, provided the airway has been protected against aspiration. Signs and symptoms of central nervous system stimulation, including convulsions, should be treated with diazepam, given slowly intravenously. Phenothiazine derivatives and central nervous system stimulants should be avoided. Hypotension and vascular collapse should be treated with intravenous fluids and, if necessary, blood pressure titration with an intravenous infusion of dilute pressor agent. It should be noted that adrenergic agents may produce a markedly increased pressor response.
Respiration should be supported by appropriate measures, including management of the airway, use of supplemental oxygen, and mechanical ventilatory assistance, as required.
Body temperature should be monitored closely. Intensive management of hyperpyrexia may be required. Maintenance of fluid and electrolyte balance is essential.
There are no data on the lethal dose in man. The pathophysiologic effects of massive overdosage may persist for several days, since the drug acts by inhibiting physiologic enzyme systems. With symptomatic and supportive measures, recovery from mild overdosage may be expected within 3 to 4 days.
Hemodialysis, peritoneal dialysis, and charcoal hemoperfusion may be of value in massive overdosage, but sufficient data are not available to recommend their routine use in these cases.
Toxic blood levels of phenelzine have not been established, and assay methods are not practical for clinical or toxicological use.
-
HOW SUPPLIED
Each Phenelzine Sulfate Tablets is orange, biconvex, film-coated tablets, debossed with "NL" on one side and "360" on the other side. Contains phenelzine sulfate equivalent to 15 mg of phenelzine base.
NDC: 43386-360-21. Bottle of 60
Storage
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Preserve in tight containers, protected from heat and light.
Rx only
Manufactured by:
Novel Laboratories Inc,
Somerset, NJ 08873
Manufactured for:
Lupin Pharmaceuticals, Inc.
Baltimore, MD 21202
PI3600000204
Rev. 05/2016
-
MEDICATION GUIDE
Antidepressant Medicines, Depression and other Serious Mental Illnesses, And Suicidal Thoughts or Actions
Read the Medication Guide that comes with you or your family member's antidepressant medicine. This Medication Guide is only about the risk of suicidal thoughts and actions with antidepressant medicines. Talk to your, or your family member's, healthcare provider about:
- all risks and benefits of treatment with antidepressant medicines
- all treatment choices for depression or other serious mental illness
What is the most important information I should know about antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions?
- Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
- Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) bipolar illness (also called manic-depressive illness) or suicidal thoughts or actions.
- How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling very agitated or restless
- panic attacks
- an extreme increase in activity and talking (mania)
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- other unusual changes in behavior or mood
- Visual problems: eye pain, changes in vision, swelling or redness in or around the eye.
What else do I need to know about antidepressant medicines?
- Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
- Visual problems: Only some people are at risk for these problems. You may want to undergo an eye examination to see if you are at risk and receive preventative treatment if you are.
- Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
- Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
- Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
- Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child's healthcare provider for more information.
- Call your doctor for medical advice about side effects, You may report side effects to FDA at 1-800- FDA-1088
Medication Guide revised on May 2016
This Medication Guide has been approved by the U.S. Food and Drug Administration for all antidepressants.
Manufactured by:
Novel Laboratories, Inc.
Somerset, NJ 08873
Manufactured for:
Lupin Pharmaceuticals, Inc.
Baltimore, MD 21202
PI3600000204
Rev. 05/2016
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
PHENELZINE SULFATE
phenelzine sulfate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 43386-360 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENELZINE SULFATE (UNII: 2681D7P965) (PHENELZINE - UNII:O408N561GF) PHENELZINE 15 mg Inactive Ingredients Ingredient Name Strength MANNITOL (UNII: 3OWL53L36A) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POVIDONE (UNII: FZ989GH94E) EDETATE DISODIUM (UNII: 7FLD91C86K) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color ORANGE Score no score Shape ROUND Size 9mm Flavor Imprint Code NL;360 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43386-360-21 60 in 1 BOTTLE; Type 0: Not a Combination Product 02/14/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA200181 12/14/2010 Labeler - Lupin Pharmaceuticals,Inc. (089153071) Registrant - Novel Laboratories, Inc. (793518643) Establishment Name Address ID/FEI Business Operations Novel Laboratories, Inc. 793518643 ANALYSIS(43386-360) , MANUFACTURE(43386-360) , PACK(43386-360)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
