DESMOPRESSIN ACETATE injection, solution
desmopressin acetate by
Drug Labeling and Warnings
desmopressin acetate by is a Prescription medication manufactured, distributed, or labeled by Sagent Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Desmopressin Acetate Injection, USP 4 mcg per mL is a synthetic analogue of the natural pituitary hormone 8-arginine vasopressin (ADH), an antidiuretic hormone affecting renal water conservation. It is chemically defined as follows:
Mol. Wt. 1183.34 Empirical Formula: C46H64N14O12S2C2H4O23H2O
1-(3-mercaptopropionic acid)-8-D-arginine vasopressin monoacetate (salt) trihydrate.
Desmopressin Acetate Injection, USP 4 mcg per mL is provided as a sterile, aqueous solution for intravenous or subcutaneous injection.
Each mL provides:
- Desmopressin acetate 4 mcg
- Sodium chloride 9 mg
- Hydrochloric acid to adjust pH to 4
The 10 mL vial contains chlorobutanol as a preservative (5 mg per mL).
-
CLINICAL PHARMACOLOGY
Desmopressin acetate contains as active substance, desmopressin acetate, a synthetic analogue of the natural hormone arginine vasopressin. One mL (4 mcg) of desmopressin acetate solution has an antidiuretic activity of about 16 IU; 1 mcg of desmopressin acetate is equivalent to 4 IU.
Desmopressin acetate has been shown to be more potent than arginine vasopressin in increasing plasma levels of factor VIII activity in patients with hemophilia and von Willebrand's disease Type I.
Dose-response studies were performed in healthy persons, using doses of 0.1 to 0.4 mcg/kg body weight, infused over a 10-minute period. Maximal dose response occurred at 0.3 to 0.4 mcg/kg. The response to desmopressin acetate of factor VIII activity and plasminogen activator is dose-related, with maximal plasma levels of 300 to 400 percent of initial concentrations obtained after infusion of 0.4 mcg/kg body weight. The increase is rapid and evident within 30 minutes, reaching a maximum at a point ranging from 90 minutes to two hours. The factor VIII related antigen and ristocetin cofactor activity were also increased to a smaller degree, but still are dose-dependent.
- The biphasic half-lives of desmopressin acetate were 7.8 and 75.5 minutes for the fast and slow phases, respectively, compared with 2.5 and 14.5 minutes for lysine vasopressin, another form of the hormone. As a result, desmopressin acetate provides a prompt onset of antidiuretic action with a long duration after each administration.
- The change in structure of arginine vasopressin to desmopressin acetate has resulted in a decreased vasopressor action and decreased actions on visceral smooth muscle relative to the enhanced antidiuretic activity, so that clinically effective antidiuretic doses are usually below threshold levels for effects on vascular or visceral smooth muscle.
- When administered by injection, desmopressin acetate has an antidiuretic effect about ten times that of an equivalent dose administered intranasally.
- The bioavailability of the subcutaneous route of administration was determined qualitatively using urine output data. The exact fraction of drug absorbed by that route of administration has not been quantitatively determined.
- The percentage increase of factor VIII levels in patients with mild hemophilia A and von Willebrand's disease was not significantly different from that observed in normal healthy individuals when treated with 0.3 mcg/kg of desmopressin acetate infused over 10 minutes.
- Plasminogen activator activity increases rapidly after desmopressin acetate infusion, but there has been no clinically significant fibrinolysis in patients treated with desmopressin acetate.
- The effect of repeated desmopressin acetate administration when doses were given every 12 to 24 hours has generally shown a gradual diminution of the factor VIII activity increase noted with a single dose. The initial response is reproducible in any particular patient if there are 2 or 3 days between administrations.
Human Pharmacokinetics
Desmopressin acetate is mainly excreted in the urine. A pharmacokinetic study conducted in healthy volunteers and patients with mild, moderate, and severe renal impairment (n=24, 6 subjects in each group) receiving single dose desmopressin acetate (2 mcg) injection demonstrated a difference in desmopressin acetate terminal half-life. Terminal half-life significantly increased from 3 hours in normal healthy patients to 9 hours in patients with severe renal impairment. (See CONTRAINDICATIONS.)
-
INDICATIONS AND USAGE
Hemophilia A
Desmopressin Acetate Injection is indicated for patients with hemophilia A with factor VIII coagulant activity levels greater than 5%.
Desmopressin Acetate Injection will often maintain hemostasis in patients with hemophilia A during surgical procedures and postoperatively when administered 30 minutes prior to scheduled procedure.
Desmopressin Acetate Injection will also stop bleeding in hemophilia A patients with episodes of spontaneous or trauma-induced injuries such as hemarthroses, intramuscular hematomas or mucosal bleeding.
Desmopressin Acetate Injection is not indicated for the treatment of hemophilia A with factor VIII coagulant activity levels equal to or less than 5%, or for the treatment of hemophilia B, or in patients who have factor VIII antibodies. In certain clinical situations, it may be justified to try Desmopressin Acetate Injection in patients with factor VIII levels between 2% to 5%; however, these patients should be carefully monitored.
von Willebrand's Disease (Type I)
Desmopressin Acetate Injection is indicated for patients with mild to moderate classic von Willebrand's disease (Type I) with factor VIII levels greater than 5%. Desmopressin Acetate Injection will often maintain hemostasis in patients with mild to moderate von Willebrand's disease during surgical procedures and postoperatively when administered 30 minutes prior to the scheduled procedure.
Desmopressin Acetate Injection will usually stop bleeding in mild to moderate von Willebrand's patients with episodes of spontaneous or trauma-induced injuries such as hemarthroses, intramuscular hematomas or mucosal bleeding.
Those von Willebrand's disease patients who are least likely to respond are those with severe homozygous von Willebrand's disease with factor VIII coagulant activity and factor VIII von Willebrand factor antigen levels less than 1%. Other patients may respond in a variable fashion depending on the type of molecular defect they have. Bleeding time and factor VIII coagulant activity, ristocetin cofactor activity, and von Willebrand factor antigen should be checked during administration of Desmopressin Acetate Injection to ensure that adequate levels are being achieved.
Desmopressin Acetate Injection is not indicated for the treatment of severe classic von Willebrand's disease (Type I) and when there is evidence of an abnormal molecular form of factor VIII antigen. (See WARNINGS.)
Diabetes Insipidus
Desmopressin Acetate Injection is indicated as antidiuretic replacement therapy in the management of central (cranial) diabetes insipidus and for the management of the temporary polyuria and polydipsia following head trauma or surgery in the pituitary region. Desmopressin Acetate Injection is ineffective for the treatment of nephrogenic diabetes insipidus.
Desmopressin Acetate Injection is also available as an intranasal preparation. However, this means of delivery can be compromised by a variety of factors that can make nasal insufflation ineffective or inappropriate. These include poor intranasal absorption, nasal congestion and blockage, nasal discharge, atrophy of nasal mucosa, and severe atrophic rhinitis. Intranasal delivery may be inappropriate where there is an impaired level of consciousness. In addition, cranial surgical procedures, such as transsphenoidal hypophysectomy, create situations where an alternative route of administration is needed as in cases of nasal packing or recovery from surgery.
-
CONTRAINDICATIONS
Desmopressin acetate is contraindicated in individuals with known hypersensitivity to desmopressin acetate or to any of the components of desmopressin acetate.
Desmopressin acetate is contraindicated in patients with moderate to severe renal impairment (defined as a creatinine clearance below 50 mL/min).
Desmopressin acetate is contraindicated in patients with hyponatremia or a history of hyponatremia.
-
WARNINGS
- Very rare cases of hyponatremia have been reported from world-wide postmarketing experience in patients treated with desmopressin acetate. Desmopressin acetate is a potent antidiuretic which, when administered, may lead to water intoxication and/or hyponatremia. Unless properly diagnosed and treated hyponatremia can be fatal. Therefore, fluid restriction is recommended and should be discussed with the patient and/or guardian. Careful medical supervision is required.
- When desmopressin acetate is administered to patients who do not have need of antidiuretic hormone for its antidiuretic effect, in particular in pediatric and geriatric patients, fluid intake should be adjusted downward to decrease the potential occurrence of water intoxication and hyponatremia. (See PRECAUTIONS, Pediatric Use and Geriatric Use.) All patients receiving desmopressin acetate therapy should be observed for the following signs or symptoms associated with hyponatremia: headache, nausea/vomiting, decreased serum sodium, weight gain, restlessness, fatigue, lethargy, disorientation, depressed reflexes, loss of appetite, irritability, muscle weakness, muscle spasms or cramps and abnormal mental status such as hallucinations, decreased consciousness and confusion. Severe symptoms may include one or a combination of the following: seizure, coma and/or respiratory arrest. Particular attention should be paid to the possibility of the rare occurrence of an extreme decrease in plasma osmolality that may result in seizures which could lead to coma.
- Desmopressin acetate should not be used to treat patients with Type IIB von Willebrand's disease since platelet aggregation may be induced.
- Desmopressin acetate should be used with caution in patients with habitual or psychogenic polydipsia who may be more likely to drink excessive amounts of water, putting them at greater risk of hyponatremia.
-
PRECAUTIONS
General
For injection use only.
Desmopressin acetate has infrequently produced changes in blood pressure causing either a slight elevation in blood pressure or a transient fall in blood pressure and a compensatory increase in heart rate. The drug should be used with caution in patients with coronary artery insufficiency and/or hypertensive cardiovascular disease.
Desmopressin acetate should be used with caution in patients with conditions associated with fluid and electrolyte imbalance, such as cystic fibrosis, heart failure and renal disorders, because these patients are prone to hyponatremia.
There have been rare reports of thrombotic events following desmopressin acetate in patients predisposed to thrombus formation. No causality has been determined, however, the drug should be used with caution in these patients.
Severe allergic reactions have been reported rarely. Anaphylaxis has been reported rarely with intravenous and intranasal desmopressin acetate, including isolated cases of fatal anaphylaxis with intravenous desmopressin acetate. It is not known whether antibodies to desmopressin acetate are produced after repeated injections.
Hemophilia A
Laboratory tests for assessing patient status include levels of factor VIII coagulant, factor VIII antigen and factor VIII ristocetin cofactor (von Willebrand factor) as well as activated partial thromboplastin time. Factor VIII coagulant activity should be determined before giving desmopressin acetate for hemostasis. If factor VIII coagulant activity is present at less than 5% of normal, desmopressin acetate should not be relied on.
von Willebrand's Disease
Laboratory tests for assessing patient status include levels of factor VIII coagulant activity, factor VIII ristocetin cofactor activity, and factor VIII von Willebrand factor antigen. The skin bleeding time may be helpful in following these patients.
Diabetes Insipidus
Laboratory tests for monitoring the patient include urine volume and osmolality. In some cases, plasma osmolality may be required.
Drug Interactions
Although the pressor activity of desmopressin acetate is very low compared with the antidiuretic activity, use of doses as large as 0.3 mcg/kg of desmopressin acetate with other pressor agents should be done only with careful patient monitoring. The concomitant administration of drugs that may increase the risk of water intoxication with hyponatremia, (e.g., tricyclic antidepressants, selective serotonin re-uptake inhibitors, chlorpromazine, opiate analgesics, NSAIDs, lamotrigine and carbamazepine) should be performed with caution.
Desmopressin acetate has been used with epsilon aminocaproic acid without adverse effects.
Carcinogenicity, Mutagenicity, Impairment of Fertility
Studies with desmopressin acetate have not been performed to evaluate carcinogenic potential, mutagenic potential or effects on fertility.
Pregnancy Category B
Fertility studies have not been done. Teratology studies in rats and rabbits at doses from 0.05 to 10 mcg/kg/day (approximately 0.1 times the maximum systemic human exposure in rats and up to 38 times the maximum systemic human exposure in rabbits based on surface area, mg/m2) revealed no harm to the fetus due to desmopressin acetate. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Several publications of desmopressin acetate's use in the management of diabetes insipidus during pregnancy are available; these include a few anecdotal reports of congenital anomalies and low birth weight babies. However, no causal connection between these events and desmopressin acetate has been established. A fifteen year, Swedish epidemiologic study of the use of desmopressin acetate in pregnant women with diabetes insipidus found the rate of birth defects to be no greater than that in the general population; however, the statistical power of this study is low. As opposed to preparations containing natural hormones, desmopressin acetate in antidiuretic doses has no uterotonic action and the physician will have to weigh the therapeutic advantages against the possible risks in each case.
Nursing Mothers
There have been no controlled studies in nursing mothers. A single study in postpartum women demonstrated a marked change in plasma, but little if any change in assayable desmopressin acetate in breast milk following an intranasal dose of 10 mcg. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when desmopressin acetate is administered to a nursing woman.
Pediatric Use
Use in infants and pediatric patients will require careful fluid intake restriction to prevent possible hyponatremia and water intoxication. Fluid restriction should be discussed with the patient and/or guardian. (See WARNINGS.) Desmopressin acetate should not be used in infants less than three months of age in the treatment of hemophilia A or von Willebrand's disease; safety and effectiveness in pediatric patients under 12 years of age with diabetes insipidus have not been established.
Geriatric Use
Clinical studies of desmopressin acetate did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. Desmopressin acetate is contraindicated in patients with moderate to severe renal impairment (defined as a creatinine clearance below 50 mL/min). (See CLINICAL PHARMACOLOGY, Human Pharmacokinetics, and CONTRAINDICATIONS.)
Use of desmopressin acetate in geriatric patients will require careful fluid intake restrictions to prevent possible hyponatremia and water intoxication. Fluid restriction should be discussed with the patient.
(See WARNINGS.)
-
ADVERSE REACTIONS
Infrequently, desmopressin acetate has produced transient headache, nausea, mild abdominal cramps and vulval pain. These symptoms disappeared with reduction in dosage. Occasionally, injection of desmopressin acetate has produced local erythema, swelling or burning pain. Occasional facial flushing has been reported with the administration of desmopressin acetate. Desmopressin acetate has infrequently produced changes in blood pressure causing either a slight elevation or a transient fall and a compensatory increase in heart rate. Severe allergic reactions including anaphylaxis have been reported rarely with desmopressin acetate.
See WARNINGS for the possibility of water intoxication and hyponatremia.
Post Marketing
There have been rare reports of thrombotic events (acute cerebrovascular thrombosis, acute myocardial infarction) following desmopressin acetate in patients predisposed to thrombus formation, and rare reports of hyponatremic convulsions associated with concomitant use with the following medications: oxybutinin and imipramine.
To report SUSPECTED ADVERSE REACTIONS, contact Sagent Pharmaceuticals, Inc. at 1-866-625-1618 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
OVERDOSAGE
Signs of overdose may include confusion, drowsiness, continuing headache, problems with passing urine and rapid weight gain due to fluid retention. (See WARNINGS.) In case of overdosage, the dosage should be reduced, frequency of administration decreased, or the drug withdrawn according to the severity of the condition.
There is no known specific antidote for desmopressin acetate.
An oral LD50 has not been established. An intravenous dose of 2 mg/kg in mice demonstrated no effect.
-
DOSAGE AND ADMINISTRATION
Hemophilia A and von Willebrand's Disease (Type I)
Desmopressin acetate injection is administered as an intravenous infusion at a dose of 0.3 mcg desmopressin acetate/kg body weight diluted in sterile physiological saline and infused slowly over 15 to 30 minutes. In adults and children weighing more than 10 kg, 50 mL of diluent is recommended; in children weighing 10 kg or less, 10 mL of diluent is recommended. Blood pressure and pulse should be monitored during infusion. If desmopressin acetate injection is used preoperatively, it should be administered 30 minutes prior to the scheduled procedure.
The necessity for repeat administration of desmopressin acetate injection or use of any blood products for hemostasis should be determined by laboratory response as well as the clinical condition of the patient. The tendency toward tachyphylaxis (lessening of response) with repeated administration given more frequently than every 48 hours should be considered in treating each patient.
Fluid restriction should be observed. (See WARNINGS, PRECAUTIONS, Pediatric Use and Geriatric Use.)
Diabetes Insipidus
This formulation is administered subcutaneously or by direct intravenous injection. Desmopressin acetate injection dosage must be determined for each patient and adjusted according to the pattern of response. Response should be estimated by two parameters: adequate duration of sleep and adequate, not excessive, water turnover.
The usual dosage range in adults is 0.5 mL (2 mcg) to 1 mL (4 mcg) daily, administered intravenously or subcutaneously, usually in two divided doses. The morning and evening doses should be separately adjusted for an adequate diurnal rhythm of water turnover. For patients who have been controlled on intranasal desmopressin acetate injection and who must be switched to the injection form, either because of poor intranasal absorption or because of the need for surgery, the comparable antidiuretic dose of the injection is about one-tenth the intranasal dose.
Fluid restriction should be observed. (See WARNINGS, PRECAUTIONS, Pediatric Use and Geriatric Use.)
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Geriatric Use
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. (See CLINICAL PHARMACOLOGY, Human Pharmacokinetics, CONTRAINDICATIONS, and PRECAUTIONS, Geriatric Use.)
-
HOW SUPPLIED
Desmopressin Acetate Injection, USP is supplied as follows:
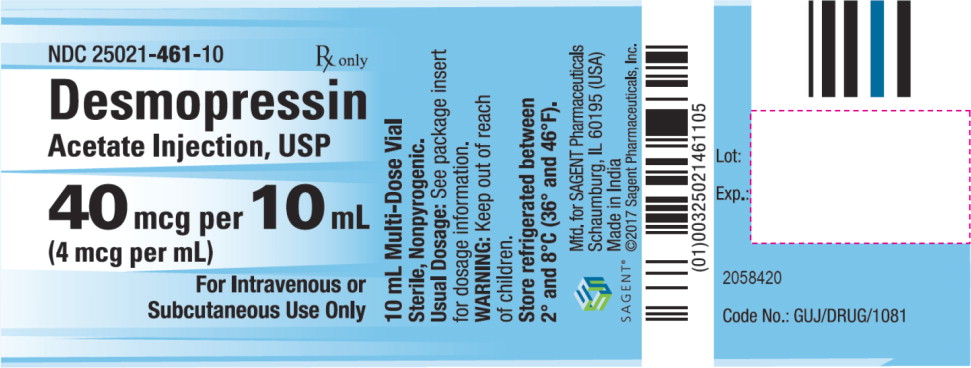
Desmopressin Acetate Injection, USP NDC (4 mcg per mL) Package Factor 25021-461-10 40 mcg per 10 mL Multi-Dose Vial 1 vial per carton Desmopressin Acetate Injection, USP is a clear, colorless solution, free from particulate matter filled in a clear glass vial.
Storage Conditions
Store refrigerated between 2° and 8°C (36° and 46°F).
Keep out of the reach of children.
Sterile, Nonpyrogenic.
The container closure is not made with natural rubber latex.
SAGENT®
Mfd. for SAGENT Pharmaceuticals
Schaumburg, IL 60195 (USA)
Made in India
©2017 Sagent Pharmaceuticals, Inc.July 2017
SAGENT Pharmaceuticals®
- PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Vial Label
-
INGREDIENTS AND APPEARANCE
DESMOPRESSIN ACETATE
desmopressin acetate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 25021-461 Route of Administration INTRAVENOUS, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength desmopressin acetate (UNII: XB13HYU18U) (desmopressin - UNII:ENR1LLB0FP) desmopressin acetate 40 ug in 10 mL Inactive Ingredients Ingredient Name Strength sodium chloride (UNII: 451W47IQ8X) hydrochloric acid (UNII: QTT17582CB) chlorobutanol (UNII: HM4YQM8WRC) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 25021-461-10 1 in 1 CARTON 03/15/2018 1 10 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204751 03/15/2018 Labeler - Sagent Pharmaceuticals (796852890)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.