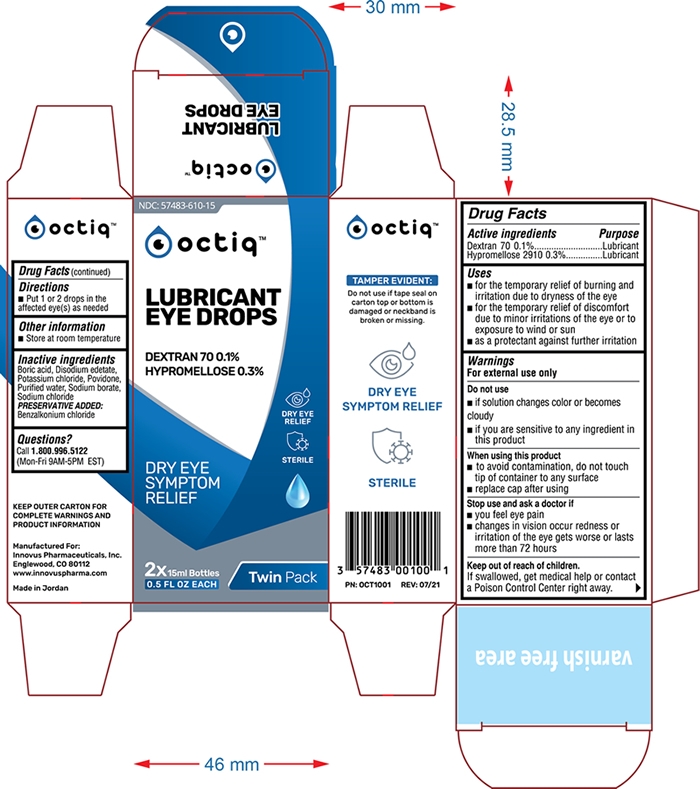
Octiq Lubricant Eye Drops
Octiq Lubricant Eye Drops by
Drug Labeling and Warnings
Octiq Lubricant Eye Drops by is a Otc medication manufactured, distributed, or labeled by Innovus Pharmaceuticals, Inc., AMMAN PHARMACEUTICAL INDUSTRIES. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
OCTIQ LUBRICANT EYE DROPS- dextran 70 hypromellose solution/ drops
Innovus Pharmaceuticals, Inc.
----------
Octiq Lubricant Eye Drops
Uses
- for the temporary relief of burning and irritation due to dryness of the eye
- for the temporary relief of discomfort due to minor irritations of the eye or to exposure to wind or sun
- as a protectant against future irritation
Do not use
- if solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
When using this product
- to avoid contamination, do not touch tip of container to any surface
- replace cap after using
Stop use and ask a doctor if
- you feel eye pain
- changes in vision occur redness or irritation of the eye gets worse or lasts more than 72 hours
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
| OCTIQ LUBRICANT EYE DROPS
dextran 70 hypromellose solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Innovus Pharmaceuticals, Inc. (962507187) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AMMAN PHARMACEUTICAL INDUSTRIES | 534677849 | manufacture(57483-610) | |
Revised: 7/2024
Document Id: 2a98ec01-c50f-45c1-83c1-fdbd3e3d53f6
Set id: 407eeffb-cb32-44bc-aef4-b1b654ac6159
Version: 4
Effective Time: 20240720