BOKA SENSITIVE- potassium nitrate paste, dentifrice
Boka Sensitive by
Drug Labeling and Warnings
Boka Sensitive by is a Otc medication manufactured, distributed, or labeled by Karaka LLC, Northwest Cosmetic Laboratories L.L.C. DBA Elevation Labs, Idaho. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
-
INACTIVE INGREDIENT
Water, Glyercin, Hydrated Silica, Sorbitol, Silica, Hydroxyapatite (nano), Sodium Lauroyl Sarcosinate, Sodium Benzoate, Xantham Gum, Xylitol, Triacertin, Flavors, Potassium Chloride, Stevia Rebaudiana Leaf/Stem Extract, Dimethyl Sulfone, Peppermint (Mentha Piperita) Oil, Sodium Bicarbonate, Spearmint (Mentha Viridis) Leaf Oil, Menthol, Wintergreen (Gaulteria Procumbens) Leaf Oil, Anise (illicium Verum) Oil, Aloe Vera (Aloe Barbadensis) Leaf Juice, Green Tea (Camellia Sinensis) Leaf Extract, Cucumber (Cucumis Sativus) Fruit Extract, Mango (Mangifera Indica) Fruit Extract, Avocado (Persea Gratissma) Fruit Extract
-
DOSAGE & ADMINISTRATION
Adults and children 12 years of age and older:Apply at least a 1-inch strip of the product onto a soft-bristled toothbrush. Brush teeth thoroughly for at least 1 minute twice a day (morning and evening), or as recommended by a dentist or doctor. Make sure to brush all sensitive areas of the teeth.
Children under 12 years: Consult a dentist or doctor.
- INDICATIONS & USAGE
-
WARNINGS
Sensitive teeth may indicate a serious problem that may need prompt care by a dentist. See your dentist if the problem persists or worsens. Do not use this product longer than 4 weeks unless recommended by a dentist or doctor.
Stop use and ask your dentistif the problem persists or worsens.
Keep out of reach of children.
If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
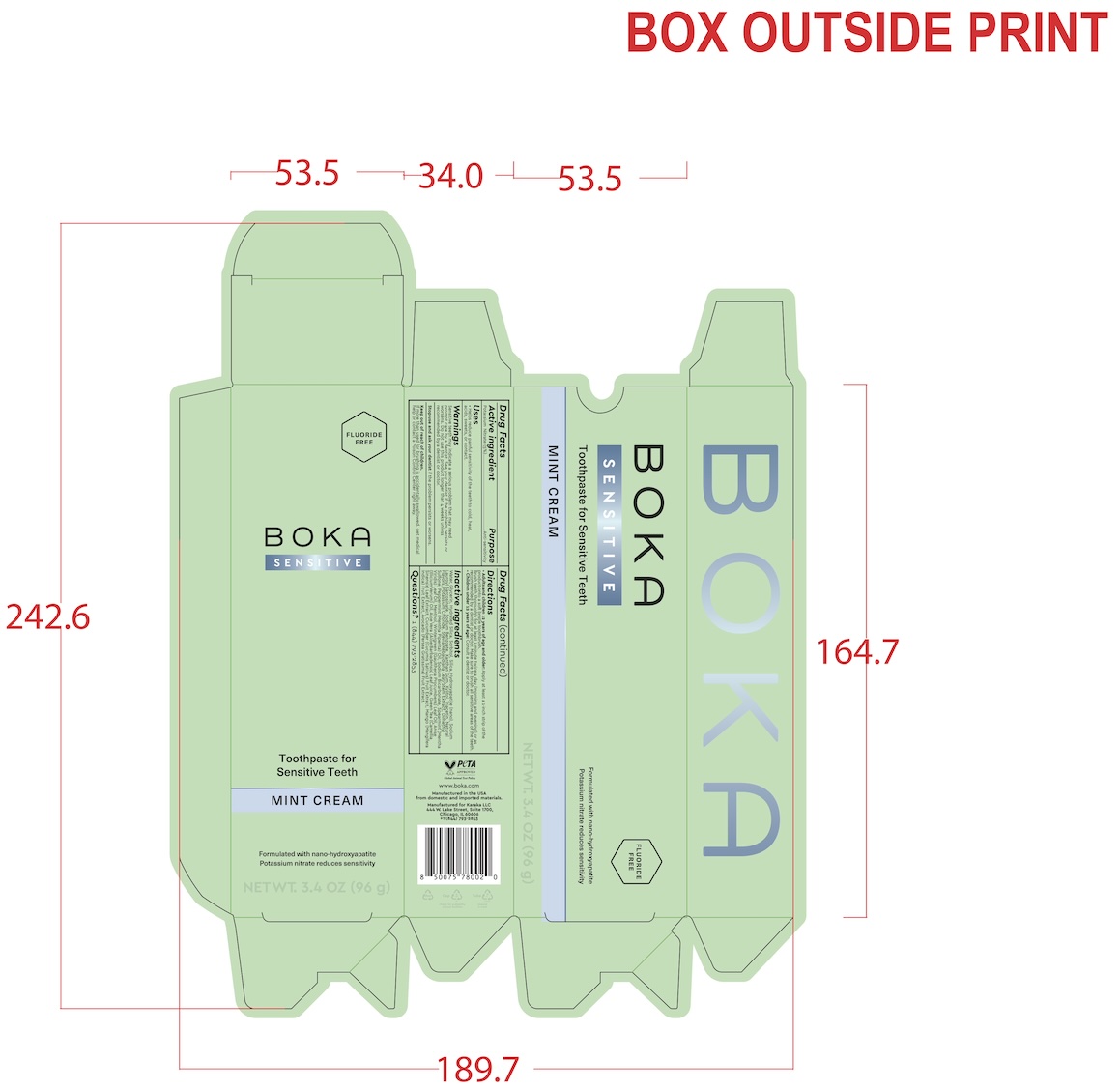
- Full Boka Sensitive 3.4oz Label Tube and Box Packaging
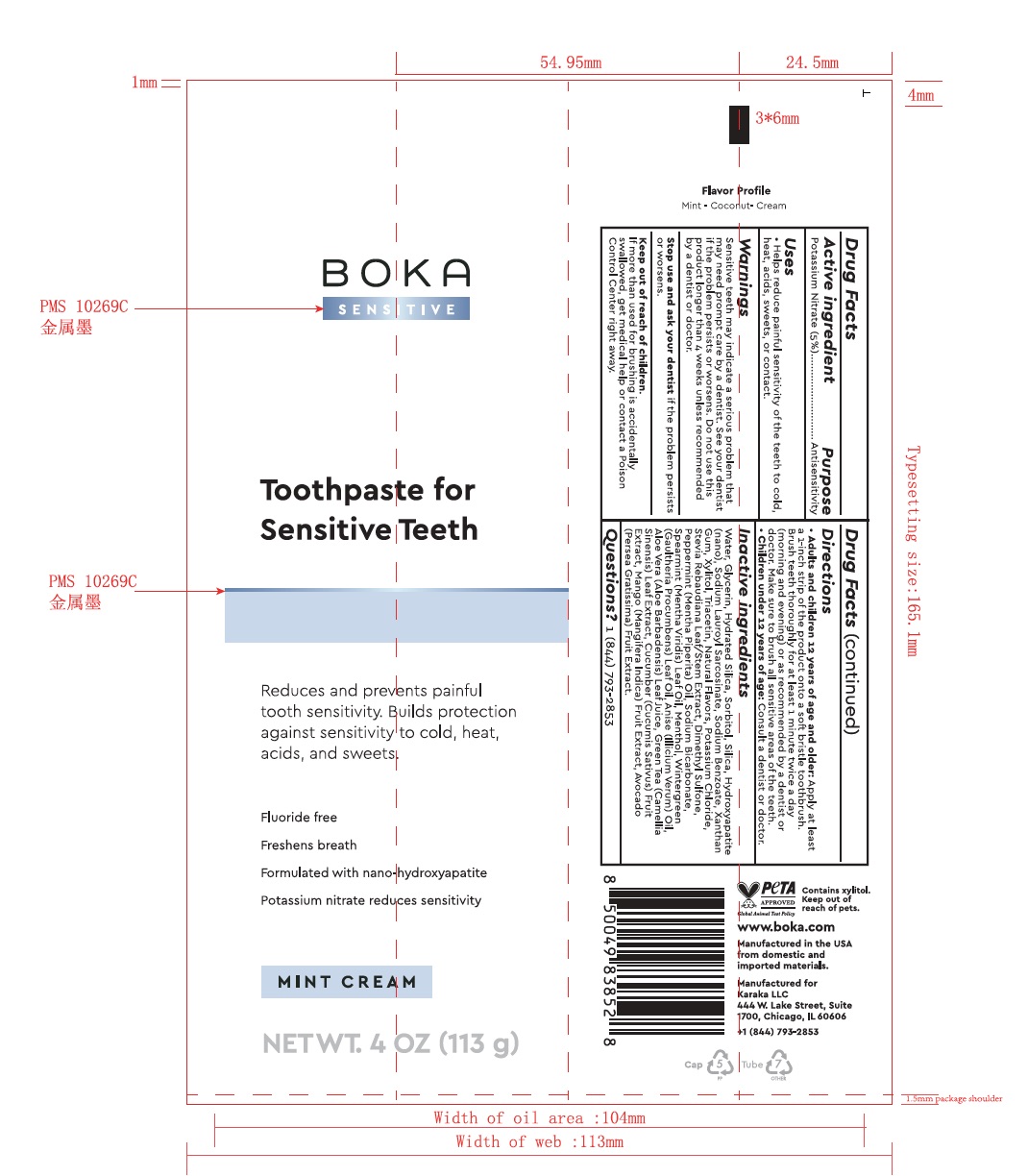
- Boka Sensitive 4.0 oz Label Tube Packaging
-
INGREDIENTS AND APPEARANCE
BOKA SENSITIVE
potassium nitrate paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 87161-444 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POTASSIUM NITRATE (UNII: RU45X2JN0Z) (NITRATE ION - UNII:T93E9Y2844) POTASSIUM NITRATE 5 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SILICA (UNII: ETJ7Z6XBU4) MENTHA VIRIDIS (SPEARMINT) LEAF OIL (UNII: C3M81465G5) GAULTHERIA PROCUMBENS (WINTERGREEN) LEAF OIL (UNII: LAV5U5022Y) HYDRATED SILICA (UNII: Y6O7T4G8P9) SODIUM BENZOATE (UNII: OJ245FE5EU) GLYCERIN (UNII: PDC6A3C0OX) SODIUM LAUROYL SARCOSINATE (UNII: 632GS99618) MENTHA PIPERITA (PEPPERMINT) OIL (UNII: AV092KU4JH) XYLITOL (UNII: VCQ006KQ1E) XANTHAN GUM (UNII: TTV12P4NEE) GREEN TEA LEAF (UNII: W2ZU1RY8B0) SODIUM BICARBONATE (UNII: 8MDF5V39QO) ALOE VERA LEAF JUICE (UNII: RUE8E6T4NB) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) SORBITOL (UNII: 506T60A25R) MANGIFERA INDICA (MANGO) FRUIT (UNII: I629I3NR86) AVOCADO (UNII: SDS87L369F) CUCUMIS SATIVUS (CUCUMBER) FRUIT (UNII: YY7C30VXJT) TRIACETIN (UNII: XHX3C3X673) STAR ANISE OIL (UNII: 6RXP35EIRE) STEVIA REBAUDIANA LEAF (UNII: 6TC6NN0876) MENTHOL (UNII: L7T10EIP3A) POTASSIUM CHLORIDE (UNII: 660YQ98I10) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 87161-444-02 1 in 1 BOX 10/09/2025 1 NDC: 87161-444-01 96 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 87161-444-03 113 g in 1 TUBE; Type 0: Not a Combination Product 10/09/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M022 10/09/2025 Labeler - Karaka LLC (118448993) Registrant - Karaka LLC (118448993) Establishment Name Address ID/FEI Business Operations Northwest Cosmetic Laboratories L.L.C. DBA Elevation Labs, Idaho 929572014 manufacture(87161-444)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.