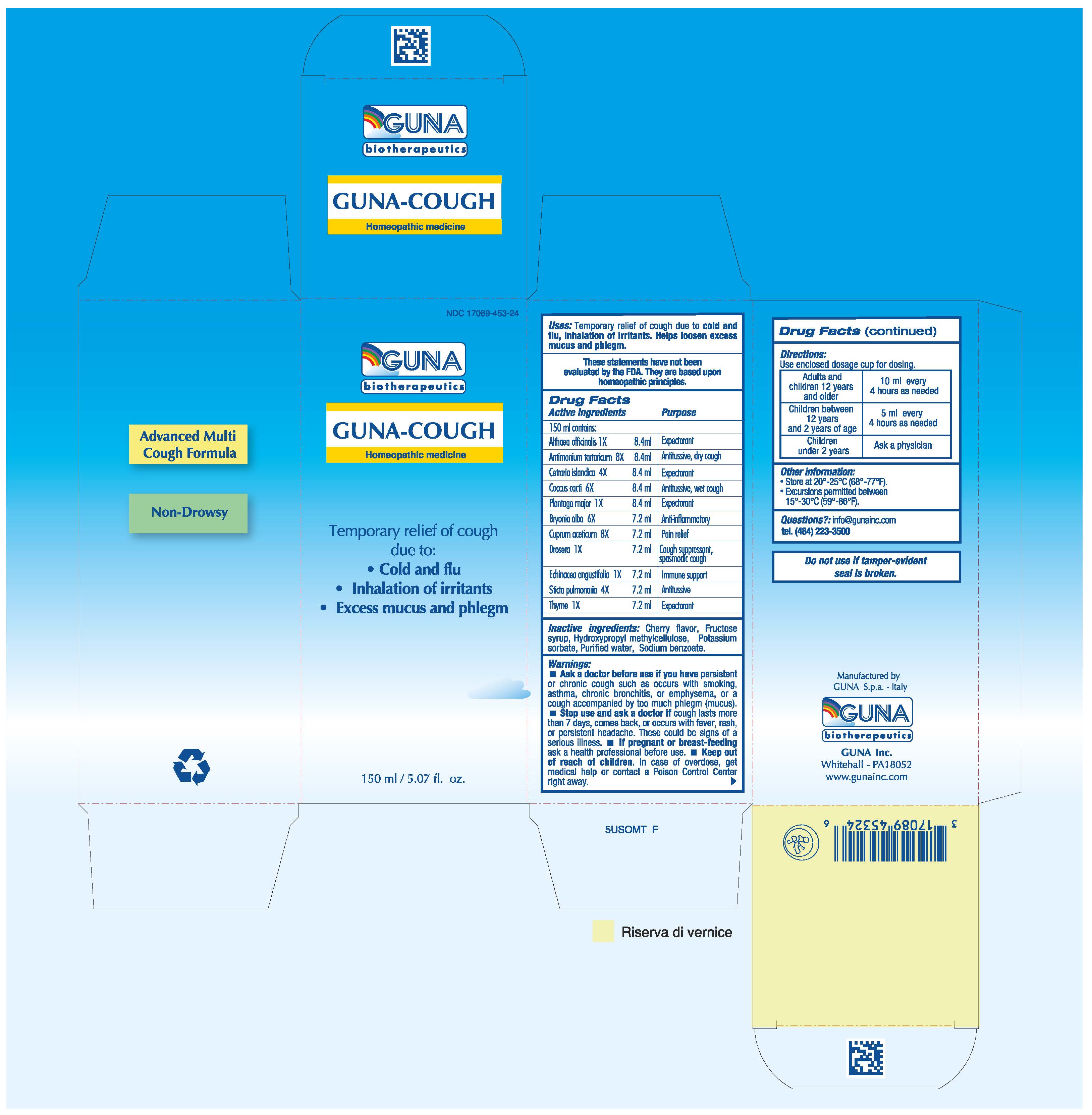
GUNA-COUGH- althaea officinalis leaf - antimony potassium tartrate - bryonia alba root - cetraria islandica subsp. islandica - cochineal - copper - drosera rotundifolia - echinacea angustifolia - garden thyme - lobaria pulmonaria - plantago major - solution/ drops
GUNA-COUGH by
Drug Labeling and Warnings
GUNA-COUGH by is a Homeopathic medication manufactured, distributed, or labeled by Guna spa. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
ACTIVE INGREDIENTS/PURPOSE
ALTHAEA OFFICINALIS 1X EXPECTORANT
ANTIMONIUM TARTARICUM 8X ANTITUSSIVE, DRY COUGH
BRYONIA ALBA 6X ANTI-INFLAMMATORY
CETRARIA ISLANDICA 4X EXPECTORANT
COCCUS CACTI 6X ANTITUSSIVE, WET COUGH
CUPRUM ACETICUM 8X PAIN RELIEF
DROSERA 1X COUGH SUPPRESSANT, SPASMODIC COUGH
ECHINACEA ANGUSTIFOLIA 1X IMMUNE SUPPORT
PLANTAGO MAJOR 1X EXPECTORANT
STICTA PULMONARIA 4X ANTITUSSIVE
THYME 1X EXPECTORANT
- USES
-
WARNINGS
Ask a doctor before use if you have persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema, or a cough accompanied by too much phlegm (mucus). Stop use and ask a doctor if cough lasts more than 7 days, comes back, or occurs with fever, rash, or persistent headache. These could be signs of a serious illness.
- PREGNANCY
- WARNINGS
- DIRECTIONS
- QUESTIONS
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GUNA-COUGH
althaea officinalis leaf - antimony potassium tartrate - bryonia alba root - cetraria islandica subsp. islandica - cochineal - copper - drosera rotundifolia - echinacea angustifolia - garden thyme - lobaria pulmonaria - plantago major - solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 17089-453 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALTHAEA OFFICINALIS LEAF (UNII: E2QQV92338) (ALTHAEA OFFICINALIS LEAF - UNII:E2QQV92338) ALTHAEA OFFICINALIS LEAF 1 [hp_X] in 150 mL ANTIMONY POTASSIUM TARTRATE (UNII: DL6OZ476V3) (ANTIMONY CATION (3+) - UNII:069647RPT5) ANTIMONY POTASSIUM TARTRATE 8 [hp_X] in 150 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 6 [hp_X] in 150 mL CETRARIA ISLANDICA SUBSP. ISLANDICA (UNII: BJ7YPN79A1) (CETRARIA ISLANDICA SUBSP. ISLANDICA - UNII:BJ7YPN79A1) CETRARIA ISLANDICA SUBSP. ISLANDICA 4 [hp_X] in 150 mL COCHINEAL (UNII: TZ8Z31B35M) (COCHINEAL - UNII:TZ8Z31B35M) COCHINEAL 6 [hp_X] in 150 mL COPPER (UNII: 789U1901C5) (COPPER - UNII:789U1901C5) COPPER 8 [hp_X] in 150 mL DROSERA ROTUNDIFOLIA (UNII: 75O014T1HG) (DROSERA ROTUNDIFOLIA - UNII:75O014T1HG) DROSERA ROTUNDIFOLIA 1 [hp_X] in 150 mL ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA 1 [hp_X] in 150 mL PLANTAGO MAJOR (UNII: W2469WNO6U) (PLANTAGO MAJOR - UNII:W2469WNO6U) PLANTAGO MAJOR 1 [hp_X] in 150 mL LOBARIA PULMONARIA (UNII: D1YM0P5Z2T) (LOBARIA PULMONARIA - UNII:D1YM0P5Z2T) LOBARIA PULMONARIA 4 [hp_X] in 150 mL THYME (UNII: CW657OBU4N) (THYME - UNII:CW657OBU4N) THYME 1 [hp_X] in 150 mL Inactive Ingredients Ingredient Name Strength FRUCTOSE (UNII: 6YSS42VSEV) 22.5 mL in 150 mL HYPROMELLOSES (UNII: 3NXW29V3WO) 2.25 mL in 150 mL POTASSIUM SORBATE (UNII: 1VPU26JZZ4) 0.15 mL in 150 mL SODIUM BENZOATE (UNII: OJ245FE5EU) 0.15 mL in 150 mL Product Characteristics Color Score Shape Size Flavor CHERRY (SOUR BLACK CHERRY) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 17089-453-24 1 in 1 BOX 04/09/2018 1 150 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 05/27/2010 Labeler - Guna spa (430538264) Establishment Name Address ID/FEI Business Operations Guna spa 338587646 manufacture(17089-453)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.