QUETIAPINE FUMARATE tablet
Quetiapine Fumarate by
Drug Labeling and Warnings
Quetiapine Fumarate by is a Prescription medication manufactured, distributed, or labeled by NuCare Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use QUETIAPINE TABLETS safely and effectively. See full prescribing information for QUETIAPINE TABLETS.
QUETIAPINE tablets, for oral use
Initial U.S. Approval: 1997WARNING: INCREASEDMORTALITYINELDERLYPATIENTS WITHDEMENTIA-RELATEDPSYCHOSIS;andSUICIDAL THOUGHTSANDBEHAVIORS
Seefullprescribing informationforcompleteboxedwarning.
IncreasedMortalityin ElderlyPatientswithDementia-Related Psychosis
· Elderly patients withdementia-relatedpsychosis treatedwith antipsychoticdrugsareatanincreasedriskofdeath.Quetiapine tablets is not approved forelderlypatients with dementia-relatedpsychosis (5.1)
SuicidalThoughtsandBehaviors
· Increasedriskofsuicidalthoughtsandbehaviorin children, adolescentsandyoungadultstakingantidepressants (5.2)
· Monitor for worsening and emergence of suicidal thoughts and behaviors (5.2)RECENT MAJOR CHANGES
WarningsandPrecautions,CerebrovascularAdverseReactions,Including Stroke,inElderlyPatientswithDementia-Related Psychosis(5.3) 4/2013
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- Quetiapine tablets, USP can betakenwithor without food (2.1)
Indication Initial Dose Recommended Dose Maximum Dose Schizophrenia-Adults (2.2) 25 mg twice daily 150 to 750 mg/day 750 mg/day Schizophrenia-Adolescents (13 to 17 years) (2.2) 25 mg twice daily 400 to 800 mg/day 800 mg/day Bipolar Mania- Adults Monotherapy or as an adjunct to lithium or divalproex (2.2) 50 mg twice daily 400 to 800 mg/day 800 mg/day Bipolar Mania- Children and Adolescents (10 to 17 years), Monotherapy (2.2) 25 mg twice daily 400 to 600 mg/day 600 mg/day Bipolar Depression-Adults (2.2) 50 mg once daily at bedtime 300 mg/day 300 mg/day DOSAGE FORMS AND STRENGTHS
Tablets: 25 mg, 50 mg, 100 mg, 150 mg, 200 mg, 300 mg, and 400 mg ( 3)
CONTRAINDICATIONS
Known hypersensitivity to quetiapine or any components in the formulation. (4)
WARNINGS AND PRECAUTIONS
· CerebrovascularAdverseReactions:Increasedincidence of cerebrovascularadverseevents(e.g.,stroke,transientischemicattack) has beenseenin elderlypatientswith dementia-relatedpsychoses treatedwithatypical antipsychotic drugs (5.3)
· NeurolepticMalignantSyndrome (NMS):Managewithimmediatediscontinuationandclosemonitoring (5.4)
· MetabolicChanges: Atypical antipsychoticshavebeenassociatedwithmetabolicchanges.Thesemetabolicchanges includehyperglycemia, dyslipidemia,andweightgain (5.5)
· HyperglycemiaandDiabetes Mellitus:Monitorpatientsforsymptomsof hyperglycemiaincludingpolydipsia,polyuria, polyphagia,andweakness.Monitorglucose regularlyinpatientswithdiabetesoratriskfor diabetes
· Dyslipidemia: Undesirablealterationshavebeenobservedin patients treatedwithatypicalantipsychotics.Appropriateclinical monitoring isrecommended,including fastingbloodlipidtesting atthebeginning of,andperiodically,duringtreatment
· WeightGain:Gain in bodyweighthas beenobserved;clinical monitoringof weightisrecommended
· TardiveDyskinesia:Discontinueifclinicallyappropriate 432 (5.6)
· Hypotension: Usewith cautionin patientswithknowncardiovascularor cerebrovascular disease (5.7)
· Increased Blood Pressure in ChildrenandAdolescents:Monitorblood pressureat thebeginning of,andperiodicallyduringtreatment inchildren andadolescents (5.8)
· Leukopenia,NeutropeniaandAgranulocytosis:Monitor completeblood countfrequentlyduring the first fewmonthsoftreatment in patientswith apre-existinglow whitecellcountorahistoryofleukopenia/neutropenia and discontinue quetiapineatthe firstsignofadeclineinWBCinabsence of othercausative factors (5.9)
· Cataracts: Lens changeshavebeenobservedin patients during long-term quetiapinetreatment.Lensexaminationisrecommendedwhenstarting treatment and at6-month intervalsduring chronictreatment (5.10)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 5% and twice placebo):
Adults:somnolence, dry mouth, dizziness, constipation, asthenia, abdominal pain,postural hypotension, pharyngitis, weight gain, lethargy, ALT increased,dyspepsia (6.1)
Childrenand Adolescents: somnolence, dizziness, fatigue, increased appetite, nausea,vomiting, dry mouth, tachycardia, weight increased (6.1)
To report SUSPECTED ADVERSE REACTIONS, contactAscend Laboratories, LLC at 1-877-ASC-RX01 (877-272-7901) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
· ConcomitantuseofstrongCYP3A4 inhibitors: Reducequetiapinedoseto onesixthwhencoadministeredwithstrong CYP3A4inhibitors(e.g., ketoconazole, ritonavir)( 2.5,7.1, 12.3)
· ConcomitantuseofstrongCYP3A4 inducers:Increasequetiapinedose upto5foldwhen used incombinationwithachronictreatment (more than7-14 days)of potentCYP3A4inducers(e.g.,phenytoin, rifampin, St.John’s wort)( 2.6, 7.1, 12.3)
· Discontinuation ofstrongCYP3A4 inducers:Reduce quetiapinedoseby5foldwithin7-14 daysofdiscontinuationofCYP3A4inducers ( 2.6, 7.1, 12.3)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2015
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS; and SUICIDAL THOUGHTS AND BEHAVIORS
1 INDICATIONS & USAGE
1.1 Schizophrenia
1.2 Bipolar Disorder
1.3 Special Considerations in Treating Pediatric Schizophrenia and Bipolar I Disorder
2 DOSAGE & ADMINISTRATION
2.1 Important Administration Instructions
2.2 Dose Modifications in Elderly Patients
2.3 Dose Modifications in Elderly Patients
2.4 Dose Modifications in Hepatically Impaired Patients
2.5 Dose Modifications when used with CYP3A4 Inhibitors
2.6 Dose Modifications when used with CYP3A4 Inducers
2.7 Reinitiation of Treatment in Patients Previously Discontinued
2.8 Switching from Antipsychotics
3 DOSAGE FORMS & STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Increased Mortality in Elderly Patients with Dementia-Related Psychosis
5.2 Suicidal Thoughts and Behaviors in Adolescents and Young Adults
5.3 Cerebrovascular Adverse Reactions, Including Stroke, in Elderly Patients with Dementia-Related Psychosis
5.4 Neuroleptic Malignant Syndrome(NMS)
5.5 Metabolic Changes
5.6 Tardive Dyskinesia
5.7 Hypotension
5.8 Increases in Blood Pressure(Children and Adolescents)
5.9 Leukopenia, Neutropenia and Agranulocytosis
5.10 Cataracts
5.11 QT Prolongation
5.12 Seizures
5.13 Hypothyroidism
5.14 Hyperprolactinemia
5.15 Potential for Cognitive and Motor Impairment
5.16 Body Temperature Regulation
5.17 Dysphagia
5.18 Discontinuation Syndrome
6 ADVERSE REACTIONS
6.1 Clinical Study Experience
6.2 Post Marketing Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on quetiapine
7.2 Effect of Quetiapine on Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
10 OVERDOSAGE
10.1 Human Experience
10.2 Management of Overdosage
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Schizophrenia
14.2 Bipolar Disorder
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS; and SUICIDAL THOUGHTS AND BEHAVIORS
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS; and SUICIDAL THOUGHTS AND BEHAVIORS
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death [ see Warnings and Precautions (5.1)]. Quetiapine is not approved for the treatment of patients with dementia-related psychosis [ see Warnings and Precautions (5.1)].
Suicidal Thoughts and Behaviors
Antidepressants increased the risk of suicidal thoughts and behavior in children, adolescents, and young adults in short-term studies. These studies did not show an increase in the risk of suicidal thoughts and behavior with antidepressant use in patients over age 24; there was a reduction in risk with antidepressant use in patients aged 65 and older [ see Warnings and Precautions (5.2)].
In patients of all ages who are started on antidepressant therapy, monitor closely for worsening, and for emergence of suicidal thoughts and behaviors. Advise families and caregivers of the need for close observation and communication with the prescriber [ see Warnings and Precautions (5.2)].
Quetiapine is not approved for use in pediatric patients under ten years of age [ see Use in Specific Populations (8.4)].
-
1 INDICATIONS & USAGE
1.1 Schizophrenia
Quetiapine is indicated for the treatment of schizophrenia. The efficacy of quetiapine in schizophrenia was established in three 6-week trials in adults and one 6-week trial in adolescents (13 to 17 years). The effectiveness of quetiapine for the maintenance treatment of schizophrenia has not been systematically evaluated in controlled clinical trials [ see Clinical Studies (14.1)].
1.2 Bipolar Disorder
Quetiapineis indicated for the acute treatment of manic episodes associated with bipolar I disorder, both as monotherapy and as an adjunct to lithium or divalproex. Efficacy was established in two 12-week monotherapy trials in adults, in one 3-week adjunctive trial in adults, and in one 3-week monotherapy trial in pediatric patients (10 to 17 years) [ see Clinical Studies (14.2)].
Quetiapine is indicated as monotherapy for the acute treatment of depressive episodes associated with bipolar disorder. Efficacy was established in two 8-week monotherapy trials in adult patients with bipolar I and bipolar II disorder [ see Clinical Studies (14.2)].
Quetiapineis indicated for the maintenance treatment of bipolar I disorder, as an adjunct to lithium or divalproex. Efficacy was established in two maintenance trials in adults. The effectiveness of quetiapine as monotherapy for the maintenance treatment of bipolar disorder has not been systematically evaluated in controlled clinical trials [ see Clinical Studies (14.2)].1.3 Special Considerations in Treating Pediatric Schizophrenia and Bipolar I Disorder
Pediatric schizophrenia and bipolar I disorder are serious mental disorders, however, diagnosis can be challenging. For pediatric schizophrenia, symptom profiles can be variable, and for bipolar I disorder, patients may have variable patterns of periodicity of manic or mixed symptoms. It is recommended that medication therapy for pediatric schizophrenia and bipolar I disorder be initiated only after a thorough diagnostic evaluation has been performed and careful consideration given to the risks associated with medication treatment. Medication treatment for both pediatric schizophrenia and bipolar I disorder is indicated as part of a total treatment program that often includes psychological, educational and social interventions.
-
2 DOSAGE & ADMINISTRATION
Quetiapine tablets can be taken with or without food.
2.2 Dose Modifications in Elderly Patients
Therecommendedinitialdose,titration,dose range and maximum quetiapine dose foreachapproved indication is displayedinTable 1. Afterinitialdosing,adjustmentscanbe madeupwardsordownwards,ifnecessary, dependingupon theclinicalresponseandtolerabilityofthepatient [ seeClinicalStudies(14.1 and 14.2)].
Indication
Initial Dose and Titration
Recommended Dose
Maximum Dose
Schizophrenia-Adults
Day 1: 25 mg twice daily. Increase in increments of 25 mg-50 mg divided two or three times on Days 2 and 3 to range of 300 to 400 mg by Day 4.
Further adjustments can be made in increments of 25 to 50 mg twice a day, in intervals of not less than 2 days.
150 to 750 mg/day
750 mg/day
Schizophrenia- Adolescents (13 to 17 years)
Day 1: 25 mg twice daily. Day 2: Twice daily dosing totaling 100 mg.
Day 3: Twice daily dosing totaling 200 mg.
Day 4: Twice daily dosing totaling 300 mg.
Day 5: Twice daily dosing totaling 400 mg.
Further adjustments should be in increments no greater than 100 mg/day within the recommended dose range of 400-800 mg/day.
Based on response and tolerability, may be administered three times daily.
400 to 800 mg/day
800 mg/day
Schizophrenia-Maintenance
N/A1
400 to 800 mg/day
800 mg/day
Bipolar Mania- Adults
Monotherapy or as an adjunct to lithium or divalproex
Day 1: Twice daily dosing totaling 100 mg.
Day 2: Twice daily dosing totaling 200 mg.
Day 3: Twice daily dosing totaling 300 mg.
Day 4: Twice daily dosing totaling 400 mg.
Further dosage adjustments up to 800 mg/day by Day 6 should be in increments of no greater than 200 mg/day.
400 to 800 mg/day
800 mg/day
Bipolar Mania- Children and Adolescents (10 to 17 years),
Monotherapy
Day 1: 25 mg twice daily.
Day 2: Twice daily dosing totaling 100 mg.
Day 3: Twice daily dosing totaling 200 mg.
Day 4: Twice daily dosing totaling 300 mg.
Day 5: Twice daily dosing totaling 400 mg.
Further adjustments should be in increments no greater than 100 mg/day within the recommended dose range of 400-600 mg/day.
Based on response and tolerability, may be administered three times daily.
400 to 600 mg/day
600 mg/day
Bipolar Depression- Adults
Administer once daily at bedtime.
Day 1: 50 mg Day 2: 100 mg Day 3: 200 mg Day 4: 300 mg
300 mg/day
300 mg/day
Bipolar I Disorder Maintenance Therapy- Adults
Administer twice daily totaling 400-800 mg/day as adjunct to lithium or divalproex. Generally, in the maintenance phase, patients continued on the same dose on which they were stabilized.
400 to 800 mg/day
800 mg/day
MaintenanceTreatmentfor Schizophreniaand BipolarI Disorder
Maintenance Treatment–Patientsshouldbeperiodicallyreassessedto determine theneedformaintenancetreatment andtheappropriatedoseforsuch treatment [see ClinicalStudies (14.2)].
2.3 Dose Modifications in Elderly Patients
Consideration should be given to a slower rate of dose titration and a lower target dose in the elderly and in patients who are debilitated or who have a predisposition to hypotensive reactions [see Clinical Pharmacology (12.3)] . When indicated, dose escalation should be performed with caution in these patients.
Elderly patients should be started on quetiapine 50 mg/day and the dose can be increased in increments of 50 mg/day depending on the clinical response and tolerability of the individual patient.2.4 Dose Modifications in Hepatically Impaired Patients
Patients with hepatic impairment should be started on 25 mg/day. The dose should be increased daily in increments of 25 mg/day - 50 mg/day to an effective dose, depending on the clinical response and tolerability of the patient.
2.5 Dose Modifications when used with CYP3A4 Inhibitors
Quetiapine dose should be reduced to one sixth of original dose when co-medicated with a potent CYP3A4 inhibitor (e.g., ketoconazole, itraconazole, indinavir, ritonavir, nefazodone, etc.). When the CYP3A4 inhibitor is discontinued, the dose of quetiapine should be increased by 6 fold [see Clinical Pharmacology (12.3) and Drug Interactions (7.1)].
2.6 Dose Modifications when used with CYP3A4 Inducers
Quetiapine dose should be increased up to 5 fold of the original dose when used in combination with a chronic treatment (e.g., greater than 7 to 14 days) of a potent CYP3A4 inducer (e.g., phenytoin, carbamazepine, rifampin, avasimibe, St. John’s wort etc.). The dose should be titrated based on the clinical response and tolerability of the individual patient. When the CYP3A4 inducer is discontinued, the dose of quetiapine should be reduced to the original level within 7-14 days [see Clinical Pharmacology (12.3)and Drug Interactions (7.1)].
2.7 Reinitiation of Treatment in Patients Previously Discontinued
Although there are no data to specifically address re-initiation of treatment, it is recommended that when restarting therapy of patients who have been off quetiapine for more than one week, the initial dosing schedule should be followed. When restarting patients who have been off quetiapine for less than one week, gradual dose escalation may not be required and the maintenance dose may be reinitiated.
2.8 Switching from Antipsychotics
There are no systematically collected data to specifically address switching patients with schizophrenia from antipsychotics to quetiapine, or concerning concomitant administration with antipsychotics. While immediate discontinuation of the previous antipsychotic treatment may be acceptable for some patients with schizophrenia, more gradual discontinuation may be most appropriate for others. In all cases, the period of overlapping antipsychotic administration should be minimized. When switching patients with schizophrenia from depot antipsychotics, if medically appropriate, initiate quetiapine therapy in place of the next scheduled injection. The need for continuing existing EPS medication should be re-evaluated periodically.
-
3 DOSAGE FORMS & STRENGTHS
- 25 mg tablets are Peach coloured, film coated, round shape, biconvex tablets, debossed with "262" on other side and plain on other side
- 50 mg tablets are White coloured, film coated, round shape, biconvex tablets, debossed with"337" on one side and plain on other side
- 100 mg tablets are Yellow coloured film coated, round shape, biconvex tablets, debossed with "261" on one side and plain on other side
- 150 mg tablets are Off white to light yellow coloured, film coated, round shape, biconvex tablets, debossed with "353" on one side and plain on other side
- 200 mg tablets are White coloured, film coated, round shape, biconvex tablets, debossed with "260" on one side and plain on other side
- 300 mg tablets are White coloured, film coated, capsule shaped, biconvex tablets, debossed with "259" on one side and plain on other side
- 400 mg tablets are Yellow coloured, film coated, capsule shaped, biconvex tablets, debossed with ''336'' on one side and plain on other side
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analysis of 17 placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. quetiapine is not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning].
5.2 Suicidal Thoughts and Behaviors in Adolescents and Young Adults
Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18 to 24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
The pooled analyses of placebo-controlled trials in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs. placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1000 patients treated) are provided in Table 2.
Table 2: Drug-Placebo Difference in Number of Cases of Suicidality per 1000 Patients Treated
Age Range
Drug-Placebo Difference in
Number of Cases of Suicidality per 1000 Patients Treated
Increases Compared to Placebo
<18
14 additional cases
18 to 24
5 additional cases
Decreases Compared to Placebo
25 to 64
1 fewer case
≥65
6 fewer cases
No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient's presenting symptoms.
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to healthcare providers. Such monitoring should include daily observation by families and caregivers. Prescriptions for quetiapine should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose.
Screening Patients for Bipolar Disorder: A major depressive episode may be the initial presentation of bipolar disorder. It is generally believed (though not established in controlled trials) that treating such an episode with an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Whether any of the symptoms described above represent such a conversion is unknown. However, prior to initiating treatment with an antidepressant, including quetiapine, patients with depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression.
5.3 Cerebrovascular Adverse Reactions, Including Stroke, in Elderly Patients with Dementia-Related Psychosis
In placebo-controlled trials with risperidone, aripiprazole, and olanzapine in elderly subjects with dementia, there was a higher incidence of cerebrovascular adverse reactions (cerebrovascular accidents and transient ischemic attacks) including fatalities compared to placebo-treated subjects. Quetiapine is not approved for the treatment of patients with dementia- related psychosis [see also Boxed Warningand Warnings and Precautions (5.1)].
5.4 Neuroleptic Malignant Syndrome(NMS)
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with administration of antipsychotic drugs, including quetiapine. Rare cases of NMS have been reported with quetiapine. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia). Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis) and acute renal failure.
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to exclude cases where the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever and primary central nervous system (CNS) pathology.
The management of NMS should include: 1) immediate discontinuation of antipsychotic drugs and other drugs not essential to concurrent therapy; 2) intensive symptomatic treatment and medical monitoring; and 3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for NMS.
If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. The patient should be carefully monitored since recurrences of NMS have been reported.5.5 Metabolic Changes
Atypical antipsychotic drugs have been associated with metabolic changes that include hyperglycemia/diabetes mellitus, dyslipidemia, and body weight gain. While all of the drugs in the class have been shown to produce some metabolic changes, each drug has its own specific risk profile. In some patients, a worsening of more than one of the metabolic parameters of weight, blood glucose, and lipids was observed in clinical studies. Changes in these metabolic profiles should be managed as clinically appropriate.
Hyperglycemia and Diabetes Mellitus
Hyperglycemia, in some cases extreme and associated with ketoacidosis or hyperosmolar coma or death, has been reported in patients treated with atypical antipsychotics, including quetiapine. Assessment of the relationship between atypical antipsychotic use and glucose abnormalities is complicated by the possibility of an increased background risk of diabetes mellitus in patients with schizophrenia and the increasing incidence of diabetes mellitus in the general population. Given these confounders, the relationship between atypical antipsychotic use and hyperglycemia-related adverse reactions is not completely understood. However, epidemiological studies suggest an increased risk of treatment- emergent hyperglycemia-related adverse reactions in patients treated with the atypical antipsychotics. Precise risk estimates for hyperglycemia-related adverse reactions in patients treated with atypical antipsychotics are not available.
Patients with an established diagnosis of diabetes mellitus who are started on atypical antipsychotics should be monitored regularly for worsening of glucose control. Patients with risk factors for diabetes mellitus (e.g., obesity, family history of diabetes) who are starting treatment with atypical antipsychotics should undergo fasting blood glucose testing at the beginning of treatment and periodically during treatment. Any patient treated with atypical antipsychotics should be monitored for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness. Patients who develop symptoms of hyperglycemia during treatment with atypical antipsychotics should undergo fasting blood glucose testing. In some cases, hyperglycemia has resolved when the atypical antipsychotic was discontinued; however, some patients required continuation of anti-diabetic treatment despite discontinuation of the suspect drug.
Adults:
Table 3: Fasting Glucose – Proportion of Patients Shifting to ≥ 126 mg/dL in Short-Term (≤12 weeks) Placebo-Controlled Studies 2
Laboratory
Analyte
Category Change
(At Least Once)
from Baseline
Treatment Arm
N
Patients
n (%)
Fasting Glucose
Normal to High (<100 mg/dL to ≥126 mg/dL/)
Quetiapine
2907
71 (2.4%)
Placebo
1346
19 (1.4%)
Borderline to High
(≥100 mg/dL) and <126 mg/dL to ≥26 PJ/G/)
Quetiapine
572
67 (11.7%)
Placebo
279
33 (11.8%)
2Includes quetiapine tablets and quetiapine extended-release tablets.
In a 24-week trial (active-controlled, 115 patients treated with quetiapine) designed to evaluate glycemic status with oral glucose tolerance testing of all patients, at week 24 the incidence of a treatment-emergent post-glucose challenge glucose level ≥ 200 mg/dL was 1.7% and the incidence of a fasting treatment-emergent blood glucose level ≥126 mg/dLwas 2.6%. The mean change in fasting glucose from baseline was 3.2 mg/dL and mean change in 2 hour glucose from baseline was -1.8 mg/dL for quetiapine.
In 2 long-term placebo-controlled randomized withdrawal clinical trials for bipolar I disorder maintenance, mean
exposure of 213 days for quetiapine(646 patients) and 152 days for placebo (680 patients), the mean change in glucose from baseline was +5.0 mg/dL for quetiapine and –0.05 mg/dL for placebo. The exposure-adjusted rate of any increased blood glucose lavel (≥ 126 mg/dL) for patients more than 8 hours since a meal (however, some patients may not have been precluded from calorie intake from fluids during fasting period) was 18.0 per 100 patient years for quetiapine (10.7% of patients; n=556) and 9.5 for placebo per 100 patient years (4.6% of patients; n=581).
Children and Adolescents:
In a placebo-controlled quetiapine monotherapy study of adolescent patients (13 to 17 years of age) with schizophrenia (6 weeks duration), the mean change in fasting glucose levels for quetiapine (n=138) compared to placebo (n=67) was – 0.75 mg/dL versus –1.70 mg/dL. In a placebo-controlled quetiapine monotherapy study of children and adolescent patients (10–17 years of age) with bipolar mania (3 weeks duration), the mean change in fasting glucose level for quetiapine (n=170) compared to placebo (n=81) was 3.62 mg/dL versus –1.17 mg/dL. No patient in either study with a baseline normal fasting glucose level (<100 mg/dL) or a baseline borderline fasting glucose level (≥ 100 mg/dL and <126mg/dL) had a treatment emergent blood glucose level ≥ 126mg/dL.
In a placebo-controlled quetiapine extended-release tablets monotherapy study (8 weeks duration) of children and adolescent patients (10 to 17 years of age) with bipolar depression, in which efficacy was not established, the mean change in fasting glucose levels for quetiapine extended-release tablets (n = 60) compared to placebo (n = 62) was 1.8 mg/dL versus 1.6 mg/dL. In this study, there were no patients in the quetiapine extended-release tablets or placebo-treated groups with a baseline normal fasting glucose level (< 100 mg/dL) that had an increase in blood glucose level > 126 mg/dL. There was one patient in the quetiapine extended-release tablets group with a baseline borderline fasting glucose level (> 100 mg/dL) and (< 126 mg/dL) who had an increase in blood glucose level of > 126 mg/dL compared to zero patients in the placebo group.
Dyslipidemia
Adults:
Table 4 shows the percentage of adult patients with changes in total cholesterol, triglycerides, LDL-cholesterol and HDL- cholesterol from baseline by indication in clinical trials with Quetiapine.
Table 4: Percentage of Adult Patients with Shifts in Total Cholesterol, Triglycerides, LDL-Cholesterol and HDL-Cholesterol from Baseline to Clinically Significant Levels by Indication
Laboratory
Analyte
Indication
Treatment Arm
N
Patients n (%)
Total Cholesterol ≥240 PJ/G/
Schizophrenia 1
Quetiapine
137
24 (18%)
Placebo
92
6 (7%)
Bipolar
Depression 2
Quetiapine
463
41 (9%)
Placebo
250
15 (6%)
Triglycerides ≥200 mg/dL
Schizophrenia 1
Quetiapine
120
26 (22%)
Placebo
70
11 (16%)
Bipolar Depression 2
Quetiapine
436
59 (14%)
Placebo
232
20 (9%)
LDL- Cholesterol
≥160 mg/dL
Schizophrenia 1
Quetiapine
na 3
na 3
Placebo
na 3
na 3
Bipolar
Depression 2
Quetiapine
465
29 (6%)
Placebo
256
12 (5%)
HDL- Cholesterol
≤40 PJ/G/
Schizophrenia 1
Quetiapine
na 3
na 3
Placebo
na 3
na 3
Bipolar
Depression 2
Quetiapine
393
56 (14%)
Placebo
214
29 (14%)
1. 6 weeks duration
2. 8 weeks duration
3. Parameters not measured in the quetiapine registration studies for schizophrenia. Lipid parameters also were not measured in the bipolar mania registration studies.
Children and Adolescents:
Table 5 shows the percentage of children and adolescents with changes in total cholesterol, triglycerides, LDL-cholesterol and HDL-cholesterol from baseline in clinical trials with quetiapine.
Table 5: Percentage of Children and Adolescents with Shifts in Total Cholesterol, Triglycerides, LDL- Cholesterol and HDL-Cholesterol from Baseline to Clinically Significant Levels
Laboratory
Analyte
Indication
Treatment Arm
N
Patients
n (%)
Total Cholesterol
≥200 PJ/G/
Schizophrenia 1
Quetiapine
107
13 (12%)
Placebo
56
1 (2%)
Bipolar Mania 2
Quetiapine
159
16 (10%)
Placebo
66
2 (3%)
Triglycerides
≥150 PJ/G/
Schizophrenia 1
Quetiapine
103
17 (17%)
Placebo
51
4 (8%)
Bipolar Mania 2
Quetiapine
149
32 (22%)
Placebo
60
8 (13%)
LDL- Cholestrol ≥
130 mg/dL
Schizophrenia 1
Quetiapine
112
4 (4%)
Placebo
60
1 (2%)
Bipolar Mania 2
Quetiapine
169
13 (8%)
Placebo
74
4 (5%)
HDL- Cholestrol ≤
40 mg/dL
Schizophrenia 1
Quetiapine
104
16 (15%)
Placebo
54
10 (19%)
Bipolar Mania 2
Quetiapine
154
16 (10%)
Placebo
61
4 (7%)
1. 13 to 17 years, 6 weeks duration
2. 10 to 17 years, 3 weeks duration
In Placebo-controlled quetiapine extended-release tablets monotherapy study (8 week duration) of children and adolescent patients (10 to 17 years of age) with bipolar depression, in which efficacy was not established, the percentage of children and adolescents with shifts in total cholesterol (≥200 mg/dL), triglycerides (≥150 mg/dL), LDL-cholesterol (≥130 mg/dL) and HDL-cholesterol (≤ 40 mg/dL) from baseline to clinically significant levels were: total cholesterol 8% (7/83) for quetiapine extended-release tablets vs. 6% (5/84) for placebo; triglycerides 28% (22/80) for quetiapine extended-release tablets vs. 9% (7/82) for placebo; LDL-cholesterol 2% (2/86) for quetiapine extended-release tablets vs. 4% (3/85) for placebo and HDL-cholesterol 20% (13/65) for quetiapine extended-release tablets vs. 15% (11/74) for placebo.
Weight Gain
Increases in weight have been observed in clinical trials. Patients receiving quetiapine should receive regular monitoring of weight.
Adults:
In clinical trials with quetiapinethe following increases in weight have been reported.
Table 6: Proportion of Patients with Weight Gain ≥ 7% of Body Weight (Adults)
Vital
Sign
Indication
Treatment Arm
N
Patients
n (%)
Weight Gain ≥7% RI Body Weight
Schizophrenia 1
Quetiapine
391
89 (23%)
Placebo
206
11 (6%)
Bipolar Mania (monotherapy) 2
Quetiapine
209
44 (21%)
Placebo
198
13 (7%)
Bipolar Mania (adjunct therapy) 3
Quetiapine
196
25 (13%)
Placebo
203
8 (4%)
Bipolar Depression 4
Quetiapine
554
47 (8%)
Placebo
295
7 (2%)
1. up to 6 weeks duration
2. up to 12 weeks duration
3. up to 3 weeks duration
4. up to 8 weeks duration
Children and Adolescents:
In two clinical trials with quetiapine, one in bipolar mania and one in schizophrenia, reported increases in weight are included in table 7.
Table 7: Proportion of Patients with Weight Gain ≥ 7% of Body Weight (Children and Adolescents)
Vital
Sign
Indication
Treatment Arm
N
Patients
n (%)
Weight
Gain
≥7% RI
Body
Schizophrenia 1
Quetiapine
111
23 (21%)
Placebo
44
3 (7%)
Bipolar Mania 2
Quetiapine
157
18 (12%)
Placebo
68
0 (0%)
1. 6 weeks duration
2. 3 weeks duration
The mean change in body weight in the schizophrenia trial was 2.0 kg in the quetiapine group and -0.4 kg in the placebo group and in the bipolar mania trial it was 1.7 kg in the quetiapine group and 0.4 kg in the placebo group.
In an open-label study that enrolled patients from the above two pediatric trials, 63% of patients (241/380) completed 26 weeks of therapy with quetiapine. After 26 weeks of treatment, the mean increase in body weight was 4.4 kg. Forty-five percent of the patients gained ≥ 7% of their body weight, not adjusted for normal growth. In order to adjust for normal growth over 26 weeks an increase of at least 0.5 standard deviation from baseline in BMI was used as a measure of a clinically significant change; 18.3% of patients on quetiapine met this criterion after 26 weeks of treatment.
In clinical trials for quetiapine extended-release tablets in children and adolescents (10 to 17 years of age) with bipolar depression, in which efficacy was not established, the percentage of patients with weight gain ≥ 7% of body weight at any time was 15% (14/92) for quetiapine extended-release tablets vs. 10% (10/100) for placebo. The mean change in body weight was 1.4 kg in the quetiapine extended-release tablets group vs. 0.6 kg in the placeo group.
When treating pediatric patients with quetiapine for any indication, weight gain should be assessed against that expected for normal growth.
5.6 Tardive Dyskinesia
A syndrome of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with antipsychotic drugs, including quetiapine. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely upon prevalence estimates to predict, at the inception of antipsychotic treatment, which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
The risk of developing tardive dyskinesia and the likelihood that it will become irreversible are believed to increase as the duration of treatment and the total cumulative dose of antipsychotic drugs administered to the patient increase. However, the syndrome can develop, although much less commonly, after relatively brief treatment periods at low doses or may even arise after discontinuation of treatment.
There is no known treatment for established cases of tardive dyskinesia, although the syndrome may remit, partially or completely, if antipsychotic treatment is withdrawn. Antipsychotic treatment, itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome and thereby may possibly mask the underlying process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown.
Given these considerations, quetiapine should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients who appear to suffer
from a chronic illness that (1) is known to respond to antipsychotic drugs, and (2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically.
If signs and symptoms of tardive dyskinesia appear in a patient on quetiapine, drug discontinuation should be considered. However, some patients may require treatment with quetiapine despite the presence of the syndrome.
5.7 Hypotension
Quetiapine may induce orthostatic hypotension associated with dizziness, tachycardia and, in some patients, syncope, especially during the initial dose-titration period, probably reflecting its α 1 -adrenergic antagonist properties. Syncope was reported in 1% (28/3265) of the patients treated with quetiapine, compared with 0.2% (2/954) on placebo and about 0.4% (2/527) on active control drugs. Orthostatic hypotension, dizziness, and syncope may lead to falls.
Quetiapine should be used with particular caution in patients with known cardiovascular disease (history of myocardial infarction or ischemic heart disease, heart failure or conduction abnormalities), cerebrovascular disease or conditions which would predispose patients to hypotension (dehydration, hypovolemia and treatment with antihypertensive medications). The risk of orthostatic hypotension and syncope may be minimized by limiting the initial dose to 25 mg twice daily [see Dosage and Administration (2.2)] . If hypotension occurs during titration to the target dose, a return to the previous dose in the titration schedule is appropriate.
5.8 Increases in Blood Pressure(Children and Adolescents)
In placebo-controlled trials in children and adolescents with schizophrenia (6-week duration) or bipolar mania (3-week duration), the incidence of increases at any time in systolic blood pressure (≥200 mm Hg) was 15.2% (51/335) for quetiapine and 5.5% (9./163) for placebo; the incidence of increases at time in diastolic blood pressure (≥100 mm HG) was 40.6% (136/335) for quetiapine and 24.5% (40/163) for placebo. In the 26-week open-label clinical trial, one child with a reported history of hypertension experienced a hypertensive crisis. Blood pressure in children and adolescents should be measured at the beginning of, and periodically during treatment.
In the placebo controlled quetiapine extended-release tablets clinical trials (8 weeks duration) in children and adolescents (10 to 17years of age) with bipolar depression, in which efficacy was not established, the incidence of increases at any time in systolic blood pressure (≥ 20 mmHG) was 6.5% (6/92) for quetiapine extended-release tablets and 6.0% (6/100) for placebo; the incidence of increases at any time in diastolic blood pressure (≥ 10 mmHG) was 46.7% (43/92) for quetiapine extended-release tablets and 36.0% (36/100) for placebo.
5.9 Leukopenia, Neutropenia and Agranulocytosis
In clinical trial and postmarketing experience, events of leukopenia/neutropenia have been reported temporally related to atypical antipsychotic agents, including quetiapine. Agranulocytosis (including fatal cases) has also been reported.
Possible risk factors for leukopenia/neutropenia include pre-existing low white cell count (WBC) and history of drug induced leukopenia/neutropenia. Patients with a pre-existing low WBC or a history of drug induced leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and should discontinue quetiapine at the first sign of a decline in WBC in absence of other causative factors.
Patients with neutropenia should be carefully monitored for fever or other symptoms or signs of infection and treated promptly if such symptoms or signs occur. Patients with severe neutropenia (absolute neutrophil count <1000/mm3) should discontinue quetiapine and have their WBC followed until recovery.
5.10 Cataracts
The development of cataracts was observed in association with quetiapine treatment in chronic dog studies [see Nonclinical Toxicology (13.2)] . Lens changes have also been observed in adults, children and adolescents during long- term quetiapine treatment, but a causal relationship to quetiapine use has not been established. Nevertheless, the possibility of lenticular changes cannot be excluded at this time. Therefore, examination of the lens by methods adequate to detect cataract formation, such as slit lamp exam or other appropriately sensitive methods, is recommended at initiation of treatment or shortly thereafter, and at 6-month intervals during chronic treatment.
5.11 QT Prolongation
In clinical trials quetiapine was not associated with a persistent increase in QT intervals. However, the QT effect was not systematically evaluated in a thorough QT study. In post marketing experience, there were cases reported of QT prolongation in patients who overdosed on quetiapine [see Overdosage (10.1)], in patients with concomitant illness, and in patients taking medicines known to cause electrolyte imbalance or increase QT interval [see Drug Interactions (7.1)].
The use of quetiapine should be avoided in combination with other drugs that are known to prolong QTc including Class 1A antiarrythmics (e.g., quinidine, procainamide) or Class III antiarrythmics (e.g., amiodarone, sotalol), antipsychotic medications (e.g., ziprasidone, chlorpromazine, thioridazine), antibiotics (e.g., gatifloxacin, moxifloxacin), or any other class of medications known to prolong the QTc interval (e.g., pentamidine, levomethadyl acetate, methadone).
Quetiapine should also be avoided in circumstances that may increase the risk of occurrence of torsade de pointes and/or sudden death including (1) a history of cardiac arrhythmias such as bradycardia; (2) hypokalemia or hypomagnesemia; (3) concomitant use of other drugs that prolong the QTc interval; and (4) presence of congenital prolongation of the QT interval.
Caution should also be exercised when quetiapine is prescribed in patients with increased risk of QT prolongation (e.g., cardiovascular disease, family history of QT prolongation, the elderly, congestive heart failure and heart hypertrophy).
5.12 Seizures
During clinical trials, seizures occurred in 0.5% (20/3490) of patients treated with quetiapine compared to 0.2% (2/954) on placebo and 0.7% (4/527) on active control drugs. As with other antipsychotics, quetiapine should be used cautiously in patients with a history of seizures or with conditions that potentially lower the seizure threshold, e.g., Alzheimer’s dementia. Conditions that lower the seizure threshold may be more prevalent in a population of 65 years or older.
5.13 Hypothyroidism
Adults: Clinical trials with quetiapine demonstrated dose-related decreases in thyroid hormone levels. The reduction in total and free thyroxine (T4) of approximately 20% at the higher end of the therapeutic dose range was maximal in the first six weeks of treatment and maintained without adaptation or progression during more chronic therapy. In nearly all cases, cessation of quetiapine treatment was associated with a reversal of the effects on total and free T4, irrespective of the duration of treatment. The mechanism by which quetiapine effects the thyroid axis is unclear. If there is an effect on the hypothalamic-pituitary axis, measurement of TSH alone may not accurately reflect a patient’s thyroid status. Therefore, both TSH and free T4, in addition to clinical assessment, should be measured at baseline and at follow-up.
In the mania adjunct studies, where quetiapine was added to lithium or divalproex, 12% (24/196) of quetiapine treated patients compared to 7% (15/203) of placebo-treated patients had elevated TSH levels. Of the quetiapine treated patients with elevated TSH levels, 3 had simultaneous low free T4 levels (free T4 <0.8 LLN).
About 0.7% (26/3489) of quetiapine patients did experience TSH increases in monotherapy studies. Some patients with TSH increases needed replacement thyroid treatment.
In all quetiapine trials, the incidence of significant shifts in thyroid hormones and TSH were1: decrease in free T4 (free T4 <0.8 LLN), 2.0% (357/17513); decrease in total T4, 4.0% (75/1861); decrease in free T3, 0.4% (53/13766); decrease in total T3, 2.0% (26/1312), and increase in TSH, 4.9% (956/19412). In eight patients, where TBG was measured, levels of TBG were unchanged.
Table 8 shows the incidence of these shifts in short term placebo-controlled clinical trials.
Table 8: Incidence of shifts in thyroid hormone levels and TSH in short-term placebo-controlled clinical trials 1
Total T4
Free T4
Total T3
Free T3
TSH
Quetiapine
Placebo
Quetiapine
Placebo
Quetiapine
Placebo
Quetiapine
Placebo
Quetiapine
Placebo
3.4 % (37/1097)
0.6% (4/651)
0.7% (52/7218)
0.1% (4/3668)
0.5% (2/369)
0.0% (0/113)
0.2% (11/5673)
0.0% (1/2679)
3.2% (240/7587)
2.7% (105/3912)
1. Based on shifts from normal baseline to potentially clinically important value at anytime post-baseline. Shifts in total T4, free T4, total T3 and free T3 are defined as <0.8 x LLN (pmol/L) and shift in TSH is >5 mlU/L at any time.
In short-term placebo-controlled monotherapy trials, the incidence of reciprocal, shifts in T3 and TSH was 0.0 % for both quetiapine (1/4800) and placebo (0/2190) and for T4 and TSH the shifts were 0.1% (7/6154) for quetiapine versus 0.0% (1/3007) for placebo.
Children and Adolescents:
In acute placebo-controlled trials in children and adolescent patients with schizophrenia (6-week duration) or bipolar mania (3-week duration), the incidence of shifts for thyroid function values at any time for Quetiapine treated patients and placebo-treated patients for elevated TSH was 2.9% (8/280) vs. 0.7% (1/138), respectively and for decreased total thyroxine was 2.8% (8/289) vs. 0% (0/145, respectively). Of the Quetiapine treated patients with elevated TSH levels, 1 had simultaneous low free T4 level at end of treatment.
5.14 Hyperprolactinemia
Adults: During clinical trials with quetiapine, the incidence of shifts in prolactin levels to a clinically significant value occurred in 3.6% (158/4416) of patients treated with quetiapine compared to 2.6% (51/1968) on placebo.
Children and Adolescents:
In acute placebo-controlled trials in children and adolescent patients with bipolar mania (3-week duration) or schizophrenia (6-week duration), the incidence of shifts in prolactin levels to a value (>20 μg/L males; > 26 μg/L females at any time) was 13.4% (18/134) for quetiapine compared to 4% (3/75) for placebo in males and 8.7% (9/104) for quetiapine compared to 0% (0/39) for placebo in females.
Like other drugs that antagonize dopamine D2 receptors, quetiapine elevates prolactin levels in some patients and the elevation may persist during chronic administration. Hyperprolactinemia, regardless of etiology, may suppress hypothalamic GnRH, resulting in reduced pituitary gonadotrophin secretion. This, in turn, may inhibit reproductive function by impairing gonadal steroidogenesis in both female and male patients. Galactorrhea, amenorrhea, gynecomastia, and impotence have been reported in patients receiving prolactin-elevating compounds. Long-standing hyperprolactinemia when associated with hypogonadism may lead to decreased bone density in both female and male subjects.
Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin dependent in vitro, a factor of potential importance if the prescription of these drugs is considered in a patient with previously detected breast cancer. As is common with compounds which increase prolactin release, mammary gland, and pancreatic islet cell neoplasia (mammary adenocarcinomas, pituitary and pancreatic adenomas) was observed in carcinogenicity studies conducted in mice and rats. Neither clinical studies nor epidemiologic studies conducted to date have shown an association between chronic administration of this class of drugs and tumorigenesis in humans, but the available evidence is too limited to be conclusive [see Nonclinical Toxicology (13.1)].
5.15 Potential for Cognitive and Motor Impairment
Somnolence was a commonly reported adverse event reported in patients treated with quetiapine especially during the 3-5 day period of initial dose-titration. In schizophrenia trials, somnolence was reported in 18% (89/510) of patients on quetiapine compared to 11% (22/206) of placebo patients. In acute bipolar mania trials using quetiapine as monotherapy, somnolence was reported in 16% (34/209) of patients on quetiapine compared to 4% of placebo patients. In acute bipolar mania trials using quetiapine as adjunct therapy, somnolence was reported in 34% (66/196) of patients on quetiapine compared to 9% (19/203) of placebo patients. In bipolar depression trials, somnolence was reported in 57% (398/698) of patients on quetiapine compared to 15% (51/347) of placebo patients. Since quetiapine has the potential to impair judgment, thinking, or motor skills, patients should be cautioned about performing activities requiring mental alertness, such as operating a motor vehicle (including automobiles) or operating hazardous machinery until they are reasonably certain that quetiapine therapy does not affect them adversely. Somnolence may lead to falls.
5.16 Body Temperature Regulation
Although not reported with quetiapine, disruption of the body's ability to reduce core body temperature has been attributed to antipsychotic agents. Appropriate care is advised when prescribing quetiapine for patients who will be experiencing conditions which may contribute to an elevation in core body temperature, e.g., exercising strenuously, exposure to extreme heat, receiving concomitant medication with anticholinergic activity, or being subject to dehydration.
5.17 Dysphagia
Esophageal dysmotility and aspiration have been associated with antipsychotic drug use. Aspiration pneumonia is a common cause of morbidity and mortality in elderly patients, in particular those with advanced Alzheimer's dementia. Quetiapine and other antipsychotic drugs should be used cautiously in patients at risk for aspiration pneumonia.
5.18 Discontinuation Syndrome
Acute withdrawal symptoms, such as insomnia, nausea, and vomiting have been described after abrupt cessation of atypical antipsychotic drugs, including quetiapine.
The incidence of the individual adverse events (i.e., insomnia, nausea, headache, diarrhea, vomiting, dizziness and irritability) did not exceed 5.3% in any treatment group and usually resolved after 1 week post-discontinuation. Gradual withdrawal is advised.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Increased mortality in elderly patients with dementia-related psychosis [see Warnings and Precautions (5.1)
- Suicidal thoughts and behaviors in adolescents and young adults [see Warnings and Precautions (5.2)]
- Cerebrovascular adverse reactions, including stroke in elderly patients with dementia-related psychosis [see Warnings and Precautions 5.3]
- Neuroleptic Malignant Syndrome (NMS) [see Warnings and Precautions 5.4]
- Metabolic changes (hyperglycemia, dyslipidemia, weight gain) [see Warnings and Precautions 5.5]
- Tardive dyskinesia [see Warnings and Precautions 5.6]
- Hypotension [see Warnings and Precautions 5.7]
- Increases in blood pressure (children and adolescents) [see Warnings and Precautions 5.8]
- Leukopenia, neutropenia and agranulocytosis [see Warnings and Precautions 5.9]
- Cataracts [see Warnings and Precautions 5.10]
- QT Prolongation [see Warnings and Precautions 5.11]
- Seizures [see Warnings and Precautions 5.12]
- Hypothyroidism [see Warnings and Precautions 5.13]
- Hyperprolactinemia [ see Warnings and Precautions 5.14]
- Potential for cognitive and motor impairment [see Warnings and Precautions 5.15]
- Body temperature regulation [see Warnings and Precautions 5.16]
- Dysphagia [see Warnings and Precautions 5.17]
- Discontinuation Syndrome [see Warnings and Precautions 5.18]
6.1 Clinical Study Experience
Becauseclinicalstudiesareconductedunderwidely varyingconditions,adversereactionratesobservedintheclinical studiesof adrugcannotbedirectlycompared toratesin theclinicalstudies ofanotherdrugand maynotreflecttheratesobservedin practice.
Adults:
Theinformationbelow isderivedfroma clinicaltrialdatabaseforquetiapineconsistingofover4300 patients.This databaseincludes 698patientsexposedtoquetiapine forthetreatmentofbipolardepression,405patientsexposedto quetiapinefor the treatmentofacutebipolarmania (monotherapyand adjunct therapy),646 patientsexposedto quetiapinefor the maintenancetreatmentofbipolarIdisorderasadjunct therapy, and approximately2600patientsand/ornormalsubjectsexposedto1 ormoredosesofquetiapine for the treatmentofschizophrenia.
Of theseapproximately4300subjects,approximately 4000(2300 inschizophrenia,405 inacutebipolarmania,698 inbipolardepression,and 646forthe maintenancetreatmentofbipolarIdisorder)werepatientswhoparticipated inmultipledose effectivenesstrials,andtheirexperiencecorresponded toapproximately2400 patient-years.Theconditionsand durationoftreatmentwith quetiapinevaried greatlyandincluded(inoverlappingcategories)open-labelanddouble-blindphases ofstudies,inpatientsandoutpatients,fixed-dose and dose-titrationstudies,andshort-termorlonger-termexposure. Adversereactions wereassessedbycollectingadverse events,results ofphysicalexaminations,vitalsigns,weights,laboratoryanalyses,ECGs, andresultsofophthalmologic examinations.
Thestated frequenciesofadverse reactions represent theproportion ofindividualswho experienced, atleastonce,a treatment-emergent adversereactionofthe typelisted.
AdverseReactions Associated with Discontinuation ofTreatment inShort-Term, Placebo-ControlledTrials
Schizophrenia:Overall,therewaslittledifferencein the incidenceofdiscontinuationdueto adversereactions(4%forquetiapine vs. 3% for placebo)ina poolofcontrolledtrials. However,discontinuationsdueto somnolence(0.8%quetiapinevs. 0%placebo) andhypotension(0.4%quetiapinevs.0%placebo)wereconsideredtobedrugrelated [see Warningsand Precautions (5.7and 5.18)].
BipolarDisorder:
Mania:Overall,discontinuations dueto adverse reactions were5.7%for quetiapinevs. 5.1% for placeboin monotherapyand 3.6%forquetiapinevs. 5.9% forplacebo in adjuncttherapy.
Depression:Overall, discontinuationsduetoadverse reactionswere12.3%forquetiapine300 mg vs. 19.0% forquetiapine 600 mgand5.2% for placebo.
CommonlyObserved AdverseReactionsin Short-Term,Placebo-Controlled Trials:
Inthe acutetherapyofschizophrenia(upto 6weeks)and bipolarmania (upto12weeks) trials,the mostcommonlyobserved adversereactionsassociated withtheuseofquetiapinemonotherapy(incidenceof5%orgreater)andobserved atarateon quetiapine at least twicethatofplacebo were somnolence (18%),dizziness(11%),drymouth (9%),constipation(8%), ALT increased(5%),weightgain (5%),and dyspepsia (5%).
AdverseReactionsOccurringatanIncidenceof2%orMoreAmong quetiapineTreated Patientsin Short-Term, Placebo-ControlledTrials:
Theprescriber shouldbe awarethat the figuresinthetablesand tabulationscannotbe usedtopredict the incidence ofsideeffectsinthe courseofusualmedical practicewherepatientcharacteristicsandotherfactors differfromthose thatprevailedin theclinical trials. Similarly,thecitedfrequenciescannotbecompared withfiguresobtainedfromotherclinicalinvestigationsinvolvingdifferenttreatments,uses, and investigators.Thecited figures,however, doprovide the prescribingphysicianwithsomebasisforestimatingtherelative contributionofdrugandnondrugfactorstothe side effectincidenceinthepopulationstudied.
Table9enumeratestheincidence,roundedtothenearestpercent,ofadversereactionsthatoccurred duringacutetherapyofschizophrenia(upto6 weeks)andbipolarmania(upto12 weeks) in2%ormoreofpatientstreated withquetiapine(dosesrangingfrom75to 800mg/day)wheretheincidencein patientstreatedwith quetiapinewasgreaterthanthe incidenceinplacebo-treatedpatients.
Table9:AdverseReactionIncidencein3-to12-WeekPlacebo-ControlledClinicalTrialsfortheTreatmentofSchizophreniaandBipolarMania(Monotherapy)
Preferred Term
Quetiapine (n=719)
PLACEBO (n=404)
Headache
21%
14%
Agitation
20%
17%
Somnolence
18%
8%
Dizziness
11%
5%
Dry Mouth
9%
3%
Constipation
8%
3%
Pain
7%
5%
Tachycardia
6%
4%
Vomiting
6%
5%
Asthenia
5%
3%
Dyspepsia
5%
1%
Weight Gain
5%
1%
ALT Increased
5%
1%
Anxiety
4%
3%
Pharyngitis
4%
3%
Rash
4%
2%
Abdominal Pain
4%
1%
Postural Hypotension
4%
1%
Back Pain
3%
1%
AST Increased
3%
1%
Rhinitis
3%
1%
Fever
2%
1%
Gastroenteritis
2%
0%
Amblyopia
2%
1%
Intheacuteadjuncttherapyofbipolarmania(upto3weeks)studies,themostcommonlyobservedadversereactionsassociated withtheuseof quetiapine(incidenceof 5%or greater)andobserved atarateonquetiapineatleasttwice thatofplacebo weresomnolence(34%),drymouth (19%),asthenia(10%), constipation (10%), abdominalpain (7%),posturalhypotension (7%),pharyngitis (6%),andweightgain (6%).
Table10enumeratestheincidence,rounded tothenearestpercent, ofadversereactionsthatoccurredduringtherapy(upto 3 weeks)ofacutemaniain 2%ormoreofpatientstreated with quetiapine(dosesrangingfrom100 to 800 mg/day)usedasadjuncttherapytolithiumanddivalproexwheretheincidencein patientstreated with quetiapinewasgreater thantheincidenceinplacebo-treatedpatients.
Table10:AdverseReactionIncidencein3-WeekPlacebo-ControlledClinicalTrialsfortheTreatmentofBipolarMania(AdjunctTherapy)Preferred Term
Quetiapine (n=196)
PLACEBO (n=203)
Somnolence
34%
9%
Dry Mouth
19%
3%
Headache
17%
13%
Asthenia
10%
4%
Constipation
10%
5%
Dizziness
9%
6%
Tremor
8%
7%
Abdominal Pain
7%
3%
Postural Hypotension
7%
2%
Agitation
6%
4%
Weight Gain
6%
3%
Pharyngitis
6%
3%
Back Pain
5%
3%
Hypertonia
4%
3%
Rhinitis
4%
2%
Peripheral Edema
4%
2%
Twitching
4%
1%
Dyspepsia
4%
3%
Depression
3%
2%
Amblyopia
3%
2%
Speech Disorder
3%
1%
Hypotension
3%
1%
Hormone Level Altered
3%
0%
Heaviness
2%
1%
Infection
2%
1%
Fever
2%
1%
Hypertension
2%
1%
Tachycardia
2%
1%
Increased Appetite
2%
1%
Hypothyroidism
2%
1%
Incoordination
2%
1%
Thinking Abnormal
2%
0%
Anxiety
2%
0%
Ataxia
2%
0%
Sinusitis
2%
1%
Sweating
2%
1%
Urinary Tract Infection
2%
1%
Inbipolardepression studies(upto8 weeks), themostcommonlyobserved treatmentemergentadversereactions associatedwiththeuseofquetiapine(incidenceof5%orgreater)and observedatarateonquetiapine atleasttwice thatofplacebo weresomnolence(57%),drymouth (44%),dizziness(18%),constipation(10%),and lethargy (5%).
Table11 enumeratestheincidence,rounded tothenearestpercent, ofadversereactionsthatoccurredduringtherapy(upto 8 weeks)ofbipolardepressionin 2% ormoreofpatientstreatedwith quetiapine(doses of300and600mg/day) wheretheincidenceinpatientstreatedwithquetiapinewasgreaterthantheincidencein placebo-treatedpatients.
Table11: AdverseReactionIncidencein8-WeekPlacebo-ControlledClinicalTrialsfortheTreatmentofBipolarDepression
Preferred Term
Quetiapine (n=698)
PLACEBO (n=347)
Somnolence 3
57%
15%
Dry Mouth
44%
13%
Dizziness
18%
7%
Constipation
10%
4%
Fatigue
10%
8%
Dyspepsia
7%
4%
Vomiting
5%
4%
Increased Appetite
5%
3%
Lethargy
5%
2%
Nasal Congestion
5%
3%
Orthostatic Hypotension
4%
3%
Akathisia
4%
1%
Palpitations
4%
1%
Vision Blurred
4%
2%
Weight increased
4%
1%
Arthralgia
3%
2%
Paraesthesia
3%
2%
Cough
3%
1%
Extrapyramidal Disorder
3%
1%
Irritability
3%
1%
Dysarthria
3%
0%
Hypersomnia
3%
0%
Sinus Congestion
2%
1%
Abnormal Dreams
2%
1%
Tremor
2%
1%
Gastroesophageal Reflux Disease
2%
1%
Pain in Extremity
2%
1%
Asthenia
2%
1%
Balance Disorder
2%
1%
Hypoaesthesia
2%
1%
Dysphagia
2%
0%
Restless Legs Syndrome
2%
0%
3. Somnolencecombinesadversereactiontermssomnolenceand sedation
Explorationsfor interactionsonthe basis of gender, age,andracedid notrevealanyclinicallymeaningfuldifferencesin theadversereactionoccurrenceon thebasisofthesedemographicfactors.
DoseDependencyofAdverseReactionsinShort-Term,Placebo-ControlledTrials
Dose-relatedAdverseReactions: Spontaneouslyelicited adversereactiondatafroma studyof schizophreniacomparing fivefixeddosesof quetiapine (75 mg,150mg, 300 mg, 600mg,and 750 mg/day)to placebo wereexploredfordose- relatednessofadversereactions. Logisticregression analysesrevealed apositivedoseresponse(p<0.05)forthefollowingadversereactions:dyspepsia, abdominalpain,and weightgain.
AdverseReactionsinclinicaltrialswith quetiapineandnotlistedelsewherein thelabel:
Thefollowingadversereactionshavealso beenreportedwithquetiapine:nightmares, hypersensitivityandelevationsinserumcreatine phosphokinase (notassociatedwithNMS), galactorrhea,bradycardia(whichmayoccur atornearinitiationof treatmentandbeassociated withhypotensionand/orsyncope) decreasedplatelets,somnambulism(and otherrelated events),elevationsingamma-GTlevels,hypothermia, and priapism.
ExtrapyramidalSymptoms (EPS):
Dystonia
ClassEffect: Symptomsofdystonia,prolonged abnormalcontractionsofmuscle groups,mayoccur insusceptible individuals duringthefirstfewdaysoftreatment. Dystonicsymptoms include:spasmoftheneckmuscles,sometimesprogressingtotightness ofthethroat,swallowingdifficulty,difficultybreathing, and/orprotrusionofthe tongue. Whilethesesymptomscanoccuratlowdoses, theyoccurmore frequentlyand with greaterseveritywith highpotencyand at higher dosesoffirstgeneration antipsychoticdrugs.Anelevated riskof acutedystoniaisobserved inmales and younger agegroups.
FourmethodswereusedtomeasureEPS:(1)Simpson-Angus totalscore(meanchangefrombaseline)whichevaluatesParkinsonismand akathisia,(2) BarnesAkathisiaRatingScale (BARS) GlobalAssessmentScore,(3)incidence ofspontaneouscomplaintsofEPS (akathisia,akinesia, cogwheelrigidity,extrapyramidalsyndrome, hypertonia, hypokinesia, neckrigidity, and tremor),and (4) use ofanticholinergicmedicationstotreatemergentEPS.
Adults:Datafromone6-weekclinicaltrialofschizophreniacomparingfive fixeddosesofquetiapine(75,150, 300,
600,750 mg/day) providedevidenceforthelackof treatment-emergentextrapyramidalsymptoms(EPS)anddose- relatednessforEPS associated with quetiapine treatment. ThreemethodswereusedtomeasureEPS:(1)Simpson- Angustotalscore(meanchangefrombaseline)whichevaluatesParkinsonismandakathisia,(2) incidenceofspontaneouscomplaintsofEPS (akathisia,akinesia,cogwheelrigidity,extrapyramidalsyndrome,hypertonia,hypokinesia,neck rigidity, and tremor),and (3)useof anticholinergicmedicationstotreatemergentEPS.
InTable 12,dystoniceventincludednuchalrigidity, hypertonia,dystonia,musclerigidity,oculogyration;parkinsonism includedcogwheelrigidity,tremor,drooling, hypokinesia;akathisiaincludedakathisia, psychomotoragitation;dyskinetic event includedtardive dyskinesia, dyskinesia,choreoathetosis;andotherextrapyramidaleventincludedrestlessness,extrapyramidaldisorder,movementdisorder.
Table12: AdversereactionsassociatedwithEPS inashort-term,placebo-controlledmultiplefixed-dosePhaseIIIschizophreniatrial (6weeksduration)
Preferred Term
Quetiapine75 mg/day (N=53)
Quetiapine 150 mg/day (N=48)
Quetiapine 300 mg/day (N=52)
Quetiapine 600 mg/day (N=51)
Quetiapine 750 mg/day (N=54)
Placebo (N=51)
n
%
n
%
n
%
n
%
n
%
n
%
Dystonic event
2
3.8
2
4.2
0
0.0
2
3.9
3
5.6
4
7.8
Parkinsonism
2
3.8
0
0.0
1
1.9
1
2.0
1
1.9
4
7.8
Akathisia
1
1.9
1
2.1
0
0.0
0
0.0
1
1.9
4
7.8
Dyskinetic event
2
3.8
0
0.0
0
0.0
1
2.0
0
0.0
0
0.0
Other extrapyramidal event
2
3.8
0
0.0
3
5.8
3
5.9
1
1.9
4
7.8
Parkinsonismincidenceratesas measured bythe Simpson-Angus totalscoreforplaceboandthefivefixeddoses(75,150,
300,600,750mg/day)were:-0.6;-1.0,-1.2;-1.6;-1.8and-1.8.Therateofanticholinergicmedicationusetotreat emergentEPS forplacebo and thefivefixeddoses was:14%;11%;10%;8%;12%and 11%.
Insix additionalplacebo-controlled clinicaltrials(3inacutemaniaand3 inschizophrenia)using variabledosesof quetiapine, therewerenodifferences between the quetiapineand placebotreatmentgroupsintheincidence ofEPS,as assessed bySimpson-Angustotalscores, spontaneouscomplaintsof EPSand the useof concomitantanticholinergicmedicationsto treatEPS.
Intwoplacebo-controlled clinicaltrialsforthetreatmentofbipolardepressionusing300 mgand 600 mgofquetiapine, theincidenceofadverse reactions potentiallyrelatedtoEPS was 12% inbothdose groups and 6%inthe placebo group.
Inthese studies, theincidenceoftheindividual adversereactions(akathisia,extrapyramidaldisorder,tremor,dyskinesia, dystonia,restlessness, musclecontractionsinvoluntary, psychomotor hyperactivityand musclerigidity)were generallylowand did notexceed 4%inanytreatmentgroup.
The3 treatmentgroups weresimilarin meanchange inSAS totalscoreandBARS GlobalAssessmentscore at the endoftreatment.Theuseofconcomitant anticholinergicmedicationswasinfrequentandsimilaracrossthethreetreatment groups.
Childrenand Adolescents
Theinformationbelow isderivedfroma clinicaltrialdatabaseforquetiapineconsistingofover1000 pediatricpatients. Thisdatabase includes 677patientsexposedto quetiapinefor the treatmentofschizophrenia and393childrenand adolescents(10to 17 yearsold) exposedto quetiapineforthetreatmentofacutebipolarmania.
AdverseReactions Associated with Discontinuation ofTreatment inShort-Term,Placebo-ControlledTrialsSchizophrenia:Theincidence ofdiscontinuationduetoadversereactionsfor quetiapine-treated andplacebo-treated patients was8.2%and2.7%, respectively.Theadverse eventleadingtodiscontinuationin 1%or moreofpatientson quetiapineand ata greaterincidence than placebowas somnolence (2.7%and 0%forplacebo).
BipolarIMania:The incidenceofdiscontinuation dueto adversereactionsforquetiapine-treated andplacebo-treatedpatientswas11.4% and4.4%, respectively.Theadverse reactionsleadingto discontinuationin 2%ormoreofpatientson quetiapineand ata greaterincidence than placebowere somnolence(4.1% vs.1.1%)andfatigue (2.1%vs.0).
CommonlyObservedAdverseReactions inShort-Term,Placebo-ControlledTrials
Intherapyforschizophrenia(upto6weeks),themostcommonlyobservedadversereactionsassociatedwiththeuseof quetiapinein adolescents(incidenceof 5%orgreaterand quetiapine incidenceatleasttwicethatfor placebo) weresomnolence(34%), dizziness(12%),drymouth (7%),tachycardia(7%).
Inbipolarmaniatherapy(up to3 weeks) the mostcommonlyobservedadversereactionsassociatedwiththeuse of quetiapineinchildrenand adolescents(incidenceof5%or greater andquetiapineincidenceatleast twicethatfor placebo)weresomnolence(53%),dizziness(18%),fatigue(11%),increasedappetite(9%),nausea(8%), vomiting(8%), tachycardia(7%),drymouth (7%),and weight increased (6%).
Adverse Reactions Occurring at an Incidenceof ≥ 2% Among quetiapine Treated Patients in Short-Term, Placebo-ControlledTrials
Schizophrenia (Adolescents,13 to 17 yearsold)
Thefollowingfindingswerebasedona6-weekplacebo-controlledtrialinwhichquetiapinewasadministered ineither dosesof 400 or800 mg/day.
Table13 enumeratestheincidence,roundedto the nearestpercent,oftreatment-emergent adverse reactionsthatoccurred duringtherapy(up to 6 weeks)ofschizophreniain2%ormoreofpatientstreatedwithquetiapine (doses of400 or800 mg/day)where the incidence inpatientstreatedwithquetiapine was atleast twice the incidence inplacebo-treated patients.
Adverse eventsthatwere potentiallydose-relatedwithhigher frequencyinthe800mg group comparedtothe400 mg group includeddizziness(8%vs. 15%), drymouth(4% vs. 10%), and tachycardia(6% vs.11%).
Table13: AdverseReactionIncidenceina6-WeekPlacebo-ControlledClinicalTrial fortheTreatment ofSchizophreniainAdolescentPatients
Preferred Term
Quetiapine 400 mg (n=73)
Quetiapine 800 mg (n=74)
Placebo (n=75)
Somnolence 1
33%
35%
11%
Dizziness
8%
15%
5%
Dry Mouth
4%
10%
1%
Tachycardia 2
6%
11%
0%
Irritability
3%
5%
0%
Arthralgia
1%
3%
0%
Asthenia
1%
3%
1%
Back Pain
1%
3%
0%
Dyspnoea
0%
3%
0%
Abdominal Pain
3%
1%
0%
Anorexia
3%
1%
0%
Tooth Abscess
3%
1%
0%
Dyskinesia
3%
0%
0%
Epistaxis
3%
0%
1%
Muscle Rigidity
3%
0%
0%
1. Somnolencecombinesadversereactiontermssomnolenceand sedation.
2. Tachycardiacombinesadversereactiontermstachycardiaand sinustachycardia.
BipolarIMania(Childrenand Adolescents 10to17 years old)
Thefollowingfindingswerebased on a3-weekplacebo-controlledtrialinwhichquetiapinewasadministeredin eitherdosesof 400 or600 mg/day.
CommonlyObservedAdverseReactions
Inbipolarmaniatherapy(upto3weeks) themostcommonlyobservedadversereactionsassociatedwiththeuseofquetiapinein children andadolescents(incidenceof5%or greater andquetiapineincidence atleast twicethatfor placebo)weresomnolence(53%),dizziness(18%),fatigue(11%),increasedappetite(9%),nausea(8%), vomiting(8%), tachycardia(7%),drymouth(7%), andweight increased(6%).
Table14 enumeratestheincidence,rounded tothenearestpercent, oftreatment-emergent adverse reactionsthatoccurredduringtherapy(up to3 weeks) of bipolar maniain2%ormore of patientstreatedwithquetiapine (doses of400 or600 mg/day)where the incidence inpatientstreatedwithquetiapinewasgreaterthan theincidenceinplacebo-treated patients.
Adverseeventsthatwerepotentiallydose-relatedwithhigher frequencyinthe600mg group comparedtothe400 mg group includedsomnolence(50%vs.57%), nausea(6% vs.10%) and tachycardia(6% vs.9%).
Table14:AdverseReactionsina3-WeekPlacebo-ControlledClinicalTrial fortheTreatment ofBipolar Maniain ChildrenandAdolescentPatients
Preferred Term
Quetiapine 400 mg (n=95)
Quetiapine 600 mg (n=98)
Placebo (n=90)
Somnolence 1
50%
57%
14%
Dizziness
19%
17%
2%
Nausea
6%
10%
4%
Fatigue
14%
9%
4%
Increased Appetite
10%
9%
1%
Tachycardia 2
6%
9%
1%
Dry Mouth
7%
7%
0%
Vomiting
8%
7%
3%
Nasal Congestion
3%
6%
2%
Weight Increased
6%
6%
0%
iritability
3%
5%
1%
Pyrexia
1%
4%
1%
Aggression
1%
3%
0%
Musculoskeletal Stiffness
1%
3%
1%
Accidental Overdose
0%
2%
0%
Acne
3%
2%
0%
Arthralgia
4%
2%
1%
Lethargy
2%
2%
0%
Pallor
1%
2%
0%
Stomach Discomfort
4%
2%
1%
Syncope
2%
2%
0%
Vision Blurred
3%
2%
0%
Constipation
4%
2%
0%
Ear Pain
2%
0%
0%
Paraesthesia
2%
0%
0%
Sinus Congestion
3%
0%
0%
Thirst
2%
0%
0%
1. Somolencecombinesadversereactionstermssomnolenceand sedation.
2. Tachycardiacombinesadversereactiontermstachycardiaand sinustachycardia.
ExtrapyramidalSymptoms:
Inashort-termplacebo-controlledmonotherapytrialinadolescentpatientswithschizophrenia(6-weekduration),theaggregatedincidenceofextrapyramidalsymptomswas 12.9%(19/147) for quetiapineand 5.3%(4/75)forplacebo, though theincidenceoftheindividualadverseevents (akathisia,tremor,extrapyramidaldisorder,hypokinesia, restlessness,psychomotorhyperactivity, musclerigidity,dyskinesia)didnotexceed 4.1%in anytreatmentgroup. Ina short-termplacebo-controlledmonotherapytrialinchildrenand adolescentpatientswithbipolarmania(3-weekduration),the aggregatedincidence ofextrapyramidalsymptomswas3.6% (7/193) orquetiapine and 1.1%(1/90)forplacebo.
Table15 presentsalistingofpatientswith adversereactionspotentiallyassociated withextrapyramidalsymptoms in the short-termplacebo-controlledmonotherapytrialinadolescentpatientswithschizophrenia(6-weekduration).
InTables15– 16dystonicevent includednuchalrigidity,hypertonia,andmusclerigidity;parkinsonismincluded cogwheelrigidityandtremor;akathisiaincludedakathisiaonly; dyskinetic event includedtardive dyskinesia,dyskinesia,andchoreoathetosis;andotherextrapyramidaleventincludedrestlessnessandextrapyramidaldisorder.
Table15: AdverseReactionsAssociatedwith ExtrapyramidalSymptomsinthePlacebo-controlled
TrialinAdolescentPatientswithSchizophrenia (6-weekduration)
Preferred Term
Quetiapine 400 mg/day (N=73)
Quetiapine 800 mg/day (N=74)
All Quetiapine (N=147)
Placebo (N=75)
n
%
n
%
n
%
n
%
Dystonic event
2
2.7
0
0.0
2
1.4
0
0.0
Parkinsonism
4
5.5
4
5.4
8
5.4
2
2.7
Akathisia
3
4.1
4
5.4
7
4.8
3
4.0
Dyskinetic event
2
2.7
0
0.0
2
1.4
0
0.0
Other Extrapyramidal Event
2
2.7
2
2.7
4
2.7
0
0.0
Table16 presentsa listingofpatientswith adversereactions associatedwithextrapyramidalsymptoms in a short-termplacebo-controlled monotherapytrialin childrenand adolescentpatientswith bipolarmania (3-weekduration).
Table16: AdverseReactionsAssociatedwithExtrapyramidalSymptomsinaPlacebo-ControlledTrialinChildrenandAdolescentPatientswithBipolarI Mania(3-weekduration)
Preferred Term1
Quetiapine 400 mg/day (N=95)
Quetiapine 600 mg/day (N=98)
All Quetiapine (N=193)
Placebo (N=90)
n
%
n
%
N
%
N
%
Parkinsonism
2
2.1
1
1.0
3
1.6
1
1.1
Akathisia
1
1.0
1
1.0
2
1.0
0
0.0
Other Extrapyramidal Event
1
1.1
1
1.0
2
1.0
0
0.0
1. Therewerenoadverseexperienceswith thepreferredtermofdystonicor dyskineticevents.
OtherAdverseReactionsObservedDuringthePre-MarketingEvaluationofquetiapine
FollowingisalistofCOSTARTterms thatreflecttreatment-emergentadversereactionsasdefinedin the introductiontothe ADVERSE REACTIONS sectionreportedbypatientstreatedwithquetiapine atmultipledoses >75mg/dayduring anyphaseofatrialwithinthe premarketingdatabase ofapproximately2200 patientstreatedfor schizophrenia. Allreported reactionsareincludedexceptthosealreadylistedinthetablesorelsewhereinlabeling,thosereactionsforwhich a drugcause wasremote,and those reaction termswhich weresogeneralastobeuninformative. It is importantto emphasize that, although the reactionsreported occurredduringtreatmentwithquetiapine, theywerenotnecessarilycaused byit.
Reactionsarefurthercategorizedbybodysystemandlistedinorderofdecreasingfrequencyaccordingtothefollowingdefinitions:frequentadverse reactionsare those occurringin at least1/100 patients(onlythosenotalreadylistedin thetabulatedresultsfromplacebo-controlledtrialsappearin thislisting); infrequentadverse reactionsarethose occurringin
1/100to 1/1000patients;rare reactions arethoseoccurringinfewerthan1/1000patients.
NervousSystem: Infrequent:abnormaldreams,dyskinesia, thinkingabnormal, tardive dyskinesia,vertigo, involuntary movements,confusion,amnesia, psychosis,hallucinations,hyperkinesia,libido increased2,urinaryretention, incoordination,paranoidreaction, abnormalgait, myoclonus,delusions, manicreaction, apathy,ataxia, depersonalization, stupor,bruxism,catatonicreaction,hemiplegia; Rare:aphasia, buccoglossalsyndrome, choreoathetosis, delirium,emotionallability,euphoria, libido decreased2,neuralgia, stuttering,subduralhematoma.
Bodyas aWhole: Frequent:flusyndrome; Infrequent:neckpain, pelvicpain2suicideattempt,malaise,photosensitivity reaction, chills,face edema,moniliasis; Rare:abdomen enlarged.
DigestiveSystem: Frequent:anorexia; Infrequent:increasedsalivation,increasedappetite, gammaglutamyltranspeptidase increased, gingivitis, dysphagia, flatulence,gastroenteritis,gastritis,hemorrhoids,stomatitis,thirst,toothcaries,fecalincontinence,gastroesophagealreflux,gumhemorrhage, mouthulceration,rectalhemorrhage, tongueedema; Rare:glossitis, hematemesis,intestinalobstruction, melena,pancreatitis.
CardiovascularSystem: Infrequent:vasodilatation,QTintervalprolonged,migraine, bradycardia,cerebralischemia, irregularpulse,Twaveabnormality,bundlebranchblock,cerebrovascularaccident,deep thrombophlebitis,Twaveinversion; Rare:anginapectoris, atrialfibrillation,AV blockfirstdegree,congestiveheartfailure, STelevated,thrombophlebitis,Twaveflattening, STabnormality,increased QRS duration.
RespiratorySystem: Frequent:cough increased,dyspnea; Infrequent:pneumonia, epistaxis,asthma; Rare: hiccup, hyperventilation.
MetabolicandNutritionalSystem: Infrequent: weightloss, alkalinephosphataseincreased,hyperlipemia,alcohol intolerance, dehydration, hyperglycemia, creatinineincreased,hypoglycemia; Rare:glycosuria,gout, handedema, hypokalemia, waterintoxication.
Skin andAppendages System: Infrequent:pruritus,acne,eczema,contactdermatitis,maculopapularrash,seborrhea,skin ulcer; Rare:exfoliativedermatitis,psoriasis,skindiscoloration.
UrogenitalSystem: Infrequent:dysmenorrhea2,vaginitis2,urinaryincontinence,metrorrhagia2,impotence2,dysuria,vaginalmoniliasis2,abnormalejaculation2,cystitis,urinary frequency, amenorrhea2,femalelactation2,leukorrhea2, vaginalhemorrhage2,vulvovaginitis2,orchitis2; Rare: gynecomastia2,nocturia,polyuria,acutekidneyfailure.
SpecialSenses: Infrequent:conjunctivitis, abnormalvision, dryeyes, tinnitus,taste perversion, blepharitis, eyepain;
Rare:abnormalityof accommodation,deafness, glaucoma.
MusculoskeletalSystem: Infrequent:pathologicalfracture,myasthenia, twitching, arthralgia,arthritis,leg cramps,bone pain.
Hemic and LymphaticSystem: Infrequent:leukocytosis, anemia, ecchymosis,eosinophilia, hypochromicanemia;lymphadenopathy, cyanosis; Rare: hemolysis,thrombocytopenia.
EndocrineSystem: Infrequent:hypothyroidism, diabetesmellitus; Rare:hyperthyroidism.
2Adjustedfor gender.
Laboratory, ECGand vitalsignchanges observed in clinicalstudies
LaboratoryChanges:
NeutrophilCounts
Adults:Inplacebo-controlledmonotherapyclinicaltrials involving3368patientsonquetiapinefumarateand 1515 on placebo,theincidenceofatleastone occurrence ofneutrophilcount<1.0x 109/Lamongpatientswith a normalbaselineneutrophilcountandatleastoneavailablefollowuplaboratorymeasurementwas0.3%(10/2967)inpatientstreatedwithquetiapinefumarate,comparedto 0.1% (2/1349)inpatientstreatedwithplacebo. [seeWarningsand Precautions (5.9)].
TransaminaseElevations
Adults:Asymptomatic, transientandreversibleelevationsinserumtransaminases(primarilyALT)havebeenreported. Inschizophreniatrials inadults, theproportions ofpatientswithtransaminase elevationsof> 3times the upper limitsofthe normalreferencerangein apoolof3-to6-weekplacebo-controlledtrialswere approximately6%(29/483)for quetiapine compared to1% (3/194)for placebo.In acutebipolarmaniatrialsinadults,theproportionsof patientswithtransaminaseelevations of> 3 times theupper limitsof thenormalreferencerangein apoolof 3-to12-weekplacebo- controlledtrialswere approximately1%forboth quetiapine (3/560) andplacebo(3/294).These hepatic enzyme elevationsusuallyoccurredwithin thefirst3weeksofdrugtreatmentand promptlyreturned topre-studylevelswithongoingtreatmentwith quetiapine. In bipolardepression trials,theproportionsof patientswithtransaminaseelevations of>3timestheupperlimitsofthenormalreferencerangeintwo8-weekplacebo-controlledtrialswas1%(5/698)for quetiapine and 2% (6/347) forplacebo.
DecreasedHemoglobin
Adults:Inshort-termplacebo-controlled trials, decreases inhaemoglobin to ≤ 13 g/dL males, ≤ 12 g/dL females on at least one occasionoccurred in 8.3% (594/7155) of quetiapine-treated patients compared to 6.2 %(219/3536) of patients treated with placebo. In a database of controlled anduncontrolled clinical trials, decreases in haemoglobin to ≤ 13 g/dL males, ≤ 12g/dL females on atleast one occasion occurred in 11% (2277/20729) of quetiapinetreated patients.
InterferencewithUrineDrugScreens
Therehavebeenliteraturereportssuggestingfalse positive resultsinurine enzyme immunoassaysformethadoneand tricyclic antidepressants inpatientswhohave takenquetiapine. Cautionshouldbeexercisedinthe interpretation of positive urinedrugscreen resultsforthesedrugs, andconfirmation byalternativeanalyticaltechnique(e.g., chromatographic methods)shouldbe considered.
ECGChanges
Adults:Between-group comparisonsforpooled placebo-controlledtrialsrevealed no statisticallysignificant quetiapine/placebo differencesintheproportionsofpatientsexperiencingpotentiallyimportantchangesinECGparameters,includingQT,QTc,andPR intervals.However,the proportions ofpatientsmeetingthe criteriafor tachycardia werecomparedinfour 3-to 6-weekplacebo-controlled clinicaltrials for the treatmentofschizophrenia revealinga 1%(4/399) incidenceforquetiapine compared to0.6%(1/156)incidence forplacebo. Inacute (monotherapy) bipolar maniatrials the proportions ofpatientsmeetingthe criteriafortachycardia was0.5%(1/192)for quetiapine compared to0% (0/178)incidence forplacebo. In acutebipolarmania(adjunct)trialstheproportionsof patientsmeetingthesamecriteriawas 0.6%(1/166)for quetiapine compared to0%(0/171)incidenceforplacebo. Inbipolardepressiontrials, nopatientshadheart rateincreases to> 120beatsperminute.Quetiapine use wasassociatedwitha meanincrease inheartrate, assessedbyECG,of 7 beats per minutecompared toa mean increaseof1beatper minute amongplacebopatients.Thisslighttendencytotachycardiainadultsmay be relatedtoquetiapine'spotential forinducingorthostaticchanges [see Warnings andPrecautions( 5.7)] .
Childrenand Adolescents:
Intheacute(6 week)schizophreniatrialinadolescents, increases inheartrate(>110 bpm)occurredin 5.2%(3/73)ofpatientsreceivingquetiapine400 mgand 8.5%(5/74)ofpatientsreceivingquetiapine 800 mgcomparedto0%(0/75)of patientsreceivingplacebo. Mean increasesinheartratewere3.8bpmand 11.2bpmfor quetiapine 400 mgand800 mg groups, respectively,compared toa decreaseof3.3 bpmin theplacebogroup [seeWarningsand Precautions ( 5.7)] .
In theacute(3 week)bipolarmania trialinchildrenand adolescents,increasesinheartrate (>110bpm) occurredin 1.1%(1/89)ofpatientsreceivingquetiapine400 mgand 4.7% (4/85)ofpatientsreceivingquetiapine600 mg compared to 0%(0/98)ofpatients receivingplacebo.Meanincreases inheartratewere12.8bpmand 13.4 bpmforquetiapine400 mgand 600 mg groups,respectively,compared to a decrease of1.7 bpminthe placebogroup [seeWarningsandPrecautions ( 5.7)] .
6.2 Post Marketing Experience
The following adverse reactions were identified during post approval of quetiapine. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse reactions reported since market introduction which were temporally related to quetiapine therapy include anaphylactic reaction, cardiomyopathy, hyponatremia, myocarditis, nocturnal enuresis, pancreatitis, retrograde amnesia, rhabdomyolysis, syndrome of inappropriate antidiuretic hormone secretion (SIADH), Stevens-Johnson syndrome (SJS), and toxic epidermal necrolysis (TEN).
-
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on quetiapine
The risks of using quetiapine in combination with other drugs have not been extensively evaluated in systematic studies. Given the primary CNS effects of quetiapine, caution should be used when it is taken in combination with other centrally acting drugs. Quetiapine potentiated the cognitive and motor effects of alcohol in a clinical trial in subjects with selected psychotic disorders, and alcoholic beverages should be limited while taking quetiapine.
Quetiapine exposure is increased by the prototype CYP3A4 inhibitors (e.g., ketoconazole, itraconazole, indinavir, ritonavir, nefazodone, etc.) and decreased by the prototype CYP3A4 inducers (e.g., phenytoin, carbamazepine, rifampin, avasimibe, St. John’s wort etc.). Dose adjustment of quetiapine will be necessary if it is co-administered with potent CYP3A4 inducers or inhibitors.
CYP3A4 inhibitors:
Coadministration of ketoconazole, a potent inhibitor of cytochrome CYP3A4, resulted in significant increase in quetiapine exposure. The dose of quetiapine should be reduced to one sixth of the original dose if coadministered with a strong CYP3A4 inhibitor [see Dosage and Administration (2.5) and Clinical Pharmacology (12.3)] .
CYP3A4 inducers:
Coadministration of quetiapine and phenytoin, a CYP3A4 inducer increased the mean oral clearance of quetiapine by 5- fold. Increased doses of quetiapine up to 5 fold may be required to maintain control of symptoms of schizophrenia in patients receiving quetiapine and phenytoin, or other known potent CYP3A4 inducers [see Dosage and Administration (2.6) and Clinical Pharmacology (12.3)] . When the CYP3A4 inducer is discontinued, the dose of quetiapine should be reduced to the original level within 7-14 days [see Dosage and Administration (2.6)] .
The potential effects of several concomitant medications on quetiapine pharmacokinetics were studied [see Clinical Pharmacology ( 12.3)] .
7.2 Effect of Quetiapine on Other Drugs
Because of its potential for inducing hypotension, Quetiapine may enhance the effects of certain antihypertensive agents.
Quetiapine may antagonize the effects of levodopa and dopamine agonists.
There are no clinically relevant pharmacokinetic interactions of quetiapine on other drugs based on the CYP pathway. Quetiapine and its metabolites are non-inhibitors of major metabolizing CYP’s (1A2, 2C9, 2C19, 2D6 and 3A4).
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C:
Risk Summary
There are no adequate and well-controlled studies of quetiapine use in pregnant women. In limited published literature, there were no major malformations associated with quetiapine exposure during pregnancy. In animal studies, embryo- fetal toxicity occurred. Quetiapine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Human Data
There are limited published data on the use of quetiapine for treatment of schizophrenia and other psychiatric disorders during pregnancy. In a prospective observational study, 21 women exposed to quetiapine and other psychoactive medications during pregnancy delivered infants with no major malformations. Among 42 other infants born to pregnant women who used quetiapine during pregnancy, there were no major malformations reported (one study of 36 women, 6 case reports). Due to the limited number of exposed pregnancies, these postmarketing data do not reliably estimate the frequency or absence of adverse outcomes. Neonates exposed to antipsychotic drugs (including quetiapine), during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery. There have been reports of agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress and feeding disorder in these neonates. These complications have varied in severity; while in some cases symptoms have been self-limited, in other cases neonates have required intensive care unit support and prolonged hospitalization.
Animal Data
When pregnant rats and rabbits were exposed to quetiapine during organogenesis, there was no teratogenic effect at doses up to 2.4 times the maximum recommended human dose (MRHD) for schizophrenia of 800 mg/day based on mg/m2 body surface area. However, there was evidence of embryo-fetal toxicity, which included delays in skeletal ossification occurring at approximately 1 and 2 times the MRHD of 800 mg/day in both rats and rabbits, and an increased incidence of carpal/tarsal flexure (minor soft tissue anomaly) in rabbit fetuses at approximately 2 times the MRHD. In addition, fetal weights were decreased in both species. Maternal toxicity (observed as decreased body weights and/or death) occurred at 2 times the MRHD in rats and approximately 1-2 times the MRHD (all doses tested) in rabbits.
In a peri/postnatal reproductive study in rats, no drug-related effects were observed when pregnant dams were treated with quetiapine at doses 0.01, 0.12, and 0.24 times the MRHD of 800 mg/day based on mg/m 2 body surface area. However, in a preliminary peri/postnatal study, there were increases in fetal and pup death, and decreases in mean litter weight at 3 times the MRHD.
8.3 Nursing Mothers
Quetiapine was excreted into human milk. Because of the potential for serious adverse reactions in nursing infants from quetiapine, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother’s health.
In published case reports, the level of quetiapine in breast milk ranged form undetectable to 170 µg/L. The estimated infant dose ranged from 0.09% to 0.43% of the weight-adjusted maternal dose. Based on a limited number (N=8) of mother/infant pairs, calculated infant daily doses range from less than 0.01 mg/kg (at a maternal daily dose up to 100 mg quetiapine ) to 0.1 mg/kg (at a maternal dose of 400 mg).
8.4 Pediatric Use
In general, the adverse reactions observed in children and adolescents during the clinical trials were similar to those in the adult population with few exceptions. Increases in systolic and diastolic blood pressure occurred in children and adolescents and did not occur in adults. Orthostatic hypotension occurred more frequently in adults (4-7%) compared to children and adolescents (< 1%) [see Warnings and Precautions (5.7) and Adverse Reactions (6.1)].
Schizophrenia
The efficacy and safety of quetiapine in the treatment of schizophrenia in adolescents aged 13 to 17 years were demonstrated in one 6-week, double-blind, placebo-controlled trial [see Indications and Usage (1.1), Dosage and Administration (2.2), Adverse Reactions (6.1), and Clinical Studies (14.1)].
Safety and effectiveness of quetiapine in pediatric patients less than 13 years of age with schizophrenia have not been established.
Maintenance
The safety and effectiveness of quetiapine in the maintenance treatment of bipolar disorder has not been established in pediatric patients less than 18 years of age. The safety and effectiveness of quetiapine in the maintenance treatment of schizophrenia has not been established in any patient population, including pediatric patients.
Bipolar Mania
The efficacy and safety of quetiapine in the treatment of mania in children and adolescents ages 10 to 17 years with Bipolar I disorder was demonstrated in a 3-week, double-blind, placebo controlled, multicenter trial [see Indications and Usage (1.2), Dosage and Administration (2.3), Adverse Reactions (6.1), and Clinical Studies (14.2)] .
Safety and effectiveness of quetiapine in pediatric patients less than 10 years of age with bipolar mania have not been established.
Bipolar Depression
Safety and effectiveness of Quetiapine in pediatric patients less than 18 years of age with bipolar depression have not been established.
Some differences in the pharmacokinetics of quetiapine were noted between children/adolescents (10 to 17 years of age) and adults. When adjusted for weight, the AUC and Cmax of quetiapine were 41% and 39% lower, respectively, in children and adolescents compared to adults. The pharmacokinetics of the active metabolite, norquetiapine, were similar between children/adolescents and adults after adjusting for weight [see Clinical Pharmacology (12.3)] .
8.5 Geriatric Use
Of the approximately 3700 patients in clinical studies with quetiapine, 7% (232) were 65 years of age or over. In general, there was no indication of any different tolerability of quetiapine in the elderly compared to younger adults. Nevertheless, the presence of factors that might decrease pharmacokinetic clearance, increase the pharmacodynamic response to quetiapine, or cause poorer tolerance or orthostasis, should lead to consideration of a lower starting dose, slower titration, and careful monitoring during the initial dosing period in the elderly. The mean plasma clearance of quetiapine was reduced by 30% to 50% in elderly patients when compared to younger patients [ see Clinical Pharmacology (12.3)and Dosage and Administration (2.3) ].
8.6 Renal Impairment
Clinical experience with quetiapine in patients with renal impairment is limited [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Since quetiapine is extensively metabolized by the liver, higher plasma levels are expected in patients with hepatic impairment. In this population, a low starting dose of 25 mg/day is recommended and the dose may be increased in increments of 25 mg/day - 50 mg/day [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)] .
-
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse
Quetiapine has not been systematically studied, in animals or humans, for its potential for abuse, tolerance or physical dependence. While the clinical trials did not reveal any tendency for any drug-seeking behavior, these observations were not systematic and it is not possible to predict on the basis of this limited experience the extent to which a CNS-active drug will be misused, diverted, and/or abused once marketed. Consequently, patients should be evaluated carefully for a history of drug abuse, and such patients should be observed closely for signs of misuse or abuse of quetiapine, e.g., development of tolerance, increases in dose, drug-seeking behavior.
-
10 OVERDOSAGE
10.1 Human Experience
In clinical trials, survival has been reported in acute overdoses of up to 30 grams of quetiapine. Most patients who overdosed experienced no adverse reactions or recovered fully from the reported reactions. Death has been reported in a clinical trial following an overdose of 13.6 grams of quetiapine alone. In general, reported signs and symptoms were those resulting from an exaggeration of the drug’s known pharmacological effects, i.e., drowsiness and sedation, tachycardia and hypotension. Patients with pre-existing severe cardiovascular disease may be at an increased risk of the effects of overdose [see Warnings and Precautions (5.11)]. One case, involving an estimated overdose of 9600 mg, was associated with hypokalemia and first degree heart block. In post-marketing experience, there were cases reported of QT prolongation with overdose. There were also very rare reports of overdose of quetiapine alone resulting in death or coma.
10.2 Management of Overdosage
In case of acute overdosage, establish and maintain an airway and ensure adequate oxygenation and ventilation. Gastric lavage (after intubation, if patient is unconscious) and administration of activated charcoal together with a laxative should be considered. The possibility of obtundation, seizure or dystonic reaction of the head and neck following overdose may create a risk of aspiration with induced emesis. Cardiovascular monitoring should commence immediately and should include continuous electrocardiographic monitoring to detect possible arrhythmias. If antiarrhythmic therapy is administered, disopyramide, procainamide and quinidine carry a theoretical hazard of additive QT-prolonging effects when administered in patients with acute overdosage of quetiapine. Similarly it is reasonable to expect that the alpha- adrenergic-blocking properties of bretylium might be additive to those of quetiapine, resulting in problematic
hypotension.
There is no specific antidote to quetiapine. Therefore, appropriate supportive measures should be instituted. The possibility of multiple drug involvement should be considered. Hypotension and circulatory collapse should be treated with appropriate measures such as intravenous fluids and/or sympathomimetic agents (epinephrine and dopamine should not be used, since beta stimulation may worsen hypotension in the setting of quetiapine-induced alpha blockade). In cases of severe extrapyramidal symptoms, anticholinergic medication should be administered. Close medical supervision and monitoring should continue until the patient recovers.
-
11 DESCRIPTION
Quetiapinefumarate is a psychotropic agent belonging to a chemical class, the dibenzothiazepine derivatives. The chemical designation is 2-[2-(4-dibenzo [ b,f] [1,4]thiazepin-11-yl-1-piperazinyl) ethoxy]-ethanol fumarate (2:1) (salt). It is present in tablets as the fumarate salt. All doses and tablet strengths are expressed as milligrams of base, not as fumarate salt. Its molecular formula is C42H50N6O4S2C4H4O4 and it has a molecular weight of 883.11 (fumarate salt). The structural formula is:
Quetiapine fumarate is a white to off-white crystalline powder which is moderately soluble in water.
Quetiapine tablets, USP is supplied for oral administration as 25 mg (round peach), 50 mg (round, white), 100 mg (round yellow), 150 mg (round, off white to light yellow), 200 mg (round, white), 300 mg (capsule-shaped, white), and 400 mg (capsule-shaped, yellow) tablets.
Inactive ingredients are povidone, dibasic dicalcium phosphate dihydrate, microcrystalline cellulose, sodium starch glycolate, lactose monohydrate, magnesium stearate, hypromellose, polyethylene glycol and titanium dioxide. The 25 mg tablets contain red iron oxide and yellow iron oxide and the 100 mg, 150 mg and 400 mg tablets contain only yellow iron oxide.
The USP dissolution test is pending.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of quetiapine is unknown. However, it has been proposed that the efficacy of quetiapine in schizophrenia and its mood stabilizing properties in bipolar depression and mania are mediated through a combination of dopamine type 2 (D2) and serotonin type 2 (5HT2) antagonism. Antagonism at receptors other than dopamine and 5HT2 with similar receptor affinities may explain some of the other effects of quetiapine.
Quetiapine’s antagonism of histamine H1 receptors may explain the somnolence observed with this drug.
Quetiapine’s antagonism of adrenergic α 1 receptors may explain the orthostatic hypotension observed with dis drug.
12.2 Pharmacodynamics
Quetiapineis an antagonist at multiple neurotransmitter receptors in the brain: serotonin 5HT1A and 5HT2 (IC50s=717 & 148nM, respectively), dopamine D1 and D2 (IC50s=1268 & 329nM, respectively), histamine H1 (IC50=30nM), and adrenergic α 1 and α 2receptors (IC50s=94 & 271nM, respectively). Quetiapine has no appreciable affinity at cholinergic muscarinic and benzodiazepine receptors (IC50s>5000 nM).
Effect on QT Interval
In clinical trials quetiapine was not associated with a persistent increase in QT intervals. However, the QT effect was not systematically evaluated in a thorough QT study. In post marketing experience there were cases reported of QT prolongation in patients who overdosed on quetiapine [see Overdosage (10.1)] , in patients with concomitant illness, and in patients taking medicines known to cause electrolyte imbalance or increase QT interval.
12.3 Pharmacokinetics
Adults
Quetiapine fumarate activity is primarily due to the parent drug. The multiple-dose pharmacokinetics of quetiapine are dose-proportional within the proposed clinical dose range, and quetiapine accumulation is predictable upon multiple dosing. Elimination of quetiapine is mainly via hepatic metabolism with a mean terminal half-life of about 6 hours within the proposed clinical dose range. Steady-state concentrations are expected to be achieved within two days of dosing. Quetiapine is unlikely to interfere with the metabolism of drugs metabolized by cytochrome P450 enzymes.
Children and Adolescents
At steady-state the pharmacokinetics of the parent compound, in children and adolescents (10-17 years of age), were similar to adults. However, when adjusted for dose and weight, AUC and Cmax of the parent compound were 41% and
39% lower, respectively, in children and adolescents than in adults. For the active metabolite, norquetiapine, AUC and
Cmax were 45% and 31% higher, respectively, in children and adolescents than in adults. When adjusted for dose and weight, the pharmacokinetics of the metabolite, norquetiapine, was similar between children and adolescents and adults [see Use in Specific Populations (8.4)] .
Absorption
Quetiapine fumarate is rapidly absorbed after oral administration, reaching peak plasma concentrations in 1.5 hours. The tablet formulation is 100% bioavailable relative to solution. The bioavailability of quetiapine is marginally affected by administration with food, with Cmax and AUC values increased by 25% and 15%, respectively.
Distribution
Quetiapine is widely distributed throughout the body with an apparent volume of distribution of 10±4 L/kg. It is 83% bound to plasma proteins at therapeutic concentrations. In vitro, quetiapine did not affect the binding of warfarin or diazepam to human serum albumin. In turn, neither warfarin nor diazepam altered the binding of quetiapine.
Metabolism and Elimination
Following a single oral dose of 14C-quetiapine, less than 1% of the administered dose was excreted as unchanged drug, indicating that quetiapine is highly metabolized. Approximately 73% and 20% of the dose was recovered in the urine and feces, respectively.
Quetiapine is extensively metabolized by the liver. The major metabolic pathways are sulfoxidation to the sulfoxide metabolite and oxidation to the parent acid metabolite; both metabolites are pharmacologically inactive. In vitro studies using human liver microsomes revealed that the cytochrome P450 3A4 isoenzyme is involved in the metabolism of quetiapine to its major, but inactive, sulfoxide metabolite and in the metabolism of its active metabolite N-desalkyl quetiapine.
Age
Oral clearance of quetiapine was reduced by 40% in elderly patients (≥ 65 years, n=9) compared to young patients (n=12), and dosing adjustment may be necessary [see Dosage and Administration (2.3)].
Gender
There is no gender effect on the pharmacokinetics of quetiapine.
Race
There is no race effect on the pharmacokinetics of quetiapine.
Smoking
Smoking has no effect on the oral clearance of quetiapine.
Renal Insufficiency
Patients with severe renal impairment (Clcr=10-30 mL/min/1.73 m2, n=8) had a 25% lower mean oral clearance than normal subjects (Clcr > 80 mL/min/1.73 m2, n=8), but plasma quetiapine concentrations in the subjects with renal insufficiency were within the range of concentrations seen in normal subjects receiving the same dose. Dosage adjustment is therefore not needed in these patients [see Use in Specific Populations (8.6)] .
Hepatic Insufficiency
Hepatically impaired patients (n=8) had a 30% lower mean oral clearance of quetiapine than normal subjects. In two of the 8 hepatically impaired patients, AUC and Cmax were 3 times higher than those observed typically in healthy subjects. Since quetiapine is extensively metabolized by the liver, higher plasma levels are expected in the hepatically impaired population, and dosage adjustment may be needed [see Dosage and Administration (2.4) and Use in Specific Populations (8.7)] .
Drug-Drug Interaction Studies
The in vivo assessments of effect of other drugs on the pharmacokinetics of quetiapine are summarized in Table 17 [see Dosage and Administration (2.5 and 2.6) and Drug Interactions (7.1)] .
Table 17: The Effect of Other Drugs on the Pharmacokinetics of Quetiapine
Coadministered drug
Dose schedules
Effect on quetiapine pharmacokinetics
Coadministered drug
Quetiapine
Phenytoin
100 mg three times daily
250 mg three times daily
5 fold Increase in oral clearance
Divalproex
500 mg twice daily
150 mg twice daily
17% increase mean max plasma concentration at steady state.
No effect on absorption or mean oral clearance
Thioridazine
200 mg twice daily
300 mg twice daily
65% increase in oral clearance
Cimetidine
400 mg three times daily for 4 days
150 mg three times daily
20% decrease in mean oral clearance
Ketoconazole (potent CYP 3A4 inhibitor)
200 mg once daily for 4 days
25 mg single dose
84% decrease in oral clearance resulting in a 6.2 fold increase in AUC of quetiapine
Fluoxetine
60 mg once daily
300 mg twice daily
No change in steady state PK
Imipramine
75 mg twice daily
300 mg twice daily
No change in steady state PK
Haloperidol
7.5 mg twice daily
300 mg twice daily
No change in steady state PK
Risperidone
3 mg twice daily
300 mg twice daily
No change in steady state PK
In vitro enzyme inhibition data suggest that quetiapine and 9 of its metabolites would have little inhibitory effect on in vivo metabolism mediated by cytochromes CYP 1A2, 2C9, 2C19, 2D6 and 3A4. Quetiapine at doses of 750 mg/day did not affect the single dose pharmacokinetics of antipyrine, lithium or lorazepam (Table 18) [see Drug Interactions (7.2)] .
Table 18: The Effect of Quetiapine on the Pharmacokinetics of Other Drugs
Coadministered drug
Dose schedules
Effect on other drugs pharmacokinetics
Coadministered drug
Quetiapine
Lorazepam
2 mg, single dose
250 mg three times daily
Oral clearance of lorazepam reduced by 20%
Divalproex
500 mg twice daily
150 mg twice daily
Cmax and AUC of free valproic acid at steady- state was decreased by 10-12%
Lithium
Up to 2400 mg/day given in twice daily doses
250 mg three times daily
No effect on steady-state pharmacokinetics of lithium
Antipyrine
1 g, single dose
250 mg three times daily
No effect on clearance of antipyrine or urinary recovery of its metabolites
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies were conducted in C57BL mice and Wistar rats. Quetiapine was administered in the diet to mice at doses of 20, 75, 250, and 750 mg/kg and to rats by gavage at doses of 25, 75, and 250 mg/kg for two years. These doses are equivalent to 0.1, 0.5, 1.5, and 4.5 times the maximum human dose (MRHD) of 800 mg/day based on mg/m2 body surface area (mice) or 0.3, 1, and 3 times the MRHD based on mg/m2 body surface area (rats). There were statistically significant increases in thyroid gland follicular adenomas in male mice at doses 1.5 and 4.5 times the MRHD on mg/m2 body surface area and in male rats at a dose of 3 times the MRHD on mg/m2 body surface area. Mammary gland adenocarcinomas were statistically significantly increased in female rats at all doses tested (0.3, 1, and 3 times the MRHD on mg/m2 body surface area).
Thyroid follicular cell adenomas may have resulted from chronic stimulation of the thyroid gland by thyroid stimulating hormone (TSH) resulting from enhanced metabolism and clearance of thyroxine by rodent liver. Changes in TSH, thyroxine, and thyroxine clearance consistent with this mechanism were observed in subchronic toxicity studies in rat and mouse and in a 1-year toxicity study in rat; however, the results of these studies were not definitive. The relevance of the increases in thyroid follicular cell adenomas to human risk, through whatever mechanism, is unknown.
Antipsychotic drugs have been shown to chronically elevate prolactin levels in rodents. Serum measurements in a 1-year toxicity study showed that quetiapine increased median serum prolactin levels a maximum of 32- and 13-fold in male and female rats, respectively. Increases in mammary neoplasms have been found in rodents after chronic administration of other antipsychotic drugs and are considered to be prolactin-mediated. The relevance of this increased incidence of prolactin-mediated mammary gland tumors in rats to human risk is unknown [see Warnings and Precautions (5.14)] .
Mutagenesis
The mutagenic potential of quetiapine was tested in the in vitro Ames bacterial gene mutation assay and in the in vitro mammalian gene mutation assay in Chinese Hamster Ovary cells. The clastogenic potential of quetiapine was tested in the in vitro chromosomal aberration assay in cultured human lymphocytes and in the in vivo bone marrow micronucleus assay in rats up to 500 mg/kg which is 6 times the maximum recommended human dose on mg/m2 body surface area. Based on weight of evidence quetiapine was not mutagenic or clastogenic in these tests.
Impairment of Fertility
Quetiapine decreased mating and fertility in male Sprague-Dawley rats at oral doses of 50 and 150 mg/kg or approximately 1 and 3 times the maximum human dose (MRHD) of 800 mg/day on mg/m2 body surface area. Drug- related effects included increases in interval to mate and in the number of matings required for successful impregnation. These effects continued to be observed at 3 times the MRHD even after a two-week period without treatment. The no- effect dose for impaired mating and fertility in male rats was 25 mg/kg, or 0.3 times the MRHD dose on mg/m2 body surface area. Quetiapine adversely affected mating and fertility in female Sprague-Dawley rats at an oral dose approximately 1 times the MRHD of 800 mg/day on mg/m2 body surface area. Drug-related effects included decreases in matings and in matings resulting in pregnancy, and an increase in the interval to mate. An increase in irregular estrus cycles was observed at doses of 10 and 50 mg/kg, or approximately 0.1 and 1 times the MRHD of 800 mg/day on mg/m2 body surface area. The no-effect dose in female rats was 1 mg/kg, or 0.01 times the MRHD of 800 mg/day on mg/m2 body surface area.
13.2 Animal Toxicology and/or Pharmacology
Quetiapine caused a dose-related increase in pigment deposition in thyroid gland in rat toxicity studies which were 4 weeks in duration or longer and in a mouse 2-year carcinogenicity study. Doses were 10 to 250 mg/kg in rats and 75 to 750 mg/kg in mice; these doses are 0.1 to 3, and 0.1 to 4.5 times the maximum recommended human dose (MRHD) of 800 mg/day on mg/m2 body surface area, respectively. Pigment deposition was shown to be irreversible in rats. The identity of the pigment could not be determined, but was found to be co-localized with quetiapine in thyroid gland follicular epithelial cells. The functional effects and the relevance of this finding to human risk are unknown.
In dogs receiving quetiapine for 6 or 12 months, but not for 1 month, focal triangular cataracts occurred at the junction of posterior sutures in the outer cortex of the lens at a dose of 100 mg/kg, or 4 times the MRHD of 800 mg/day on mg/m2 body surface area. This finding may be due to inhibition of cholesterol biosynthesis by quetiapine. Quetiapine caused a dose-related reduction in plasma cholesterol levels in repeat-dose dog and monkey studies; however, there was no correlation between plasma cholesterol and the presence of cataracts in individual dogs. The appearance of delta-8- cholestanol in plasma is consistent with inhibition of a late stage in cholesterol biosynthesis in these species. There also was a 25% reduction in cholesterol content of the outer cortex of the lens observed in a special study in quetiapine treated female dogs. Drug-related cataracts have not been seen in any other species; however, in a 1-year study in monkeys, a striated appearance of the anterior lens surface was detected in 2/7 females at a dose of 225 mg/kg or 5.5 times the MRHD of 800 mg/day on mg/m2 body surface area.
-
14 CLINICAL STUDIES
14.1 Schizophrenia
Short-term Trials-Adults
The efficacy of quetiapine in the treatment of schizophrenia was established in 3 short-term (6-week) controlled trials of inpatients with schizophrenia who met DSM III-R criteria for schizophrenia. Although a single fixed dose haloperidol arm was included as a comparative treatment in one of the three trials, this single haloperidol dose group was inadequate to provide a reliable and valid comparison of quetiapine and haloperidol.
Several instruments were used for assessing psychiatric signs and symptoms in these studies, among them the Brief Psychiatric Rating Scale (BPRS), a multi-item inventory of general psychopathology traditionally used to evaluate the effects of drug treatment in schizophrenia. The BPRS psychosis cluster (conceptual disorganization, hallucinatory behavior, suspiciousness, and unusual thought content) is considered a particularly useful subset for assessing actively psychotic schizophrenic patients. A second traditional assessment, the Clinical Global Impression (CGI), reflects the impression of a skilled observer, fully familiar with the manifestations of schizophrenia, about the overall clinical state of the patient.
The results of the trials follow:
1. In a 6-week, placebo-controlled trial (n=361) (study 1) involving 5 fixed doses of quetiapine (75 mg/day, 150 mg/day, 300 mg/day, 600 mg/day and 750 mg/day given in divided doses three times per day), the 4 highest doses of quetiapine were generally superior to placebo on the BPRS total score, the BPRS psychosis cluster and the CGI severity score, with the maximal effect seen at 300 mg/day, and the effects of doses of 150 mg/day to 750 mg/day were generally indistinguishable.
2. In a 6-week, placebo-controlled trial (n=286) (study 2) involving titration of quetiapine in high (up to 750 mg/day given in divided doses three times per day) and low (up to 250 mg/day given in divided doses three times per day) doses, only the high dose quetiapine group (mean dose, 500 mg/day) was superior to placebo on the BPRS total score, the BPRS psychosis cluster, and the CGI severity score.
3. In a 6-week dose and dose regimen comparison trial (n=618) (study 3) involving two fixed doses of quetiapine (450 mg/day given in divided doses both twice daily and three times daily and 50 mg/day given in divided doses twice daily), only the 450 mg/day (225 mg given twice daily) dose group was superior to the 50 mg/day (25 mg given twice daily) quetiapine dose group on the BPRS total score, the BPRS psychosis cluster, and the CGI severity score.
The primary efficacy results of these three studies in the treatment of schizophrenia in adults is presented in Table 19. Examination of population subsets (race, gender, and age) did not reveal any differential responsiveness on the basis of race or gender, with an apparently greater effect in patients under the age of 40 years compared to those older than 40. The clinical significance of this finding is unknown.
Adolescents (ages 13 to 17)
The efficacy of quetiapine in the treatment of schizophrenia in adolescents (13 to 17 years of age) was demonstrated in a 6-week, double-blind, placebo-controlled trial (study 4). Patients who met DSM-IV diagnostic criteria for schizophrenia were randomized into one of three treatment groups: Quetiapine 400 mg/day (n = 73), Quetiapine 800 mg/day (n = 74), or placebo (n = 75). Study medication was initiated at 50 mg/day and on day 2 increased to 100 mg/per day (divided and given two or three times per day). Subsequently, the dose was titrated to the target dose of 400 mg/day or 800 mg/day using increments of 100 mg/day, divided and given two or three times daily. The primary efficacy variable was the mean change from baseline in total Positive and Negative Syndrome Scale (PANSS).
Quetiapine at 400 mg/day and 800 mg/day was superior to placebo in the reduction of PANSS total score. The primary efficacy results of this study in the treatment of schizophrenia in adolescents is presented in Table 19.
Table 19: Schizophrenia Short-Term Trials
Study
Number
Treatment Group
Primary Efficacy Endpoint: BPRS Total
Mean Baseline
Score (SD)
LS Mean Change
from Baseline (SE)
Placebo-subtracted
Difference6 (95% CI)
Study 1
Quetiapine (75 mg/day)
45.7 (10.9)
-2.2 (2.0)
-4.0 (-11.2, 3.3)
Quetiapine (150 mg/day) 4
47.2 (10.1)
-8.7 (2.1)
-10.4 (-17.8, -3.0)
Quetiapine (300 mg/day) 4
45.3 (10.9)
-8.6 (2.1)
-10.3 (-17.6, -3.0)
Quetiapine (600 mg/day) 4
43.5 (11.3)
-7.7 (2.1)
-9.4 (-16.7, -2.1)
Quetiapine (750 mg/day) 4
45.7 (11.0)
-6.3 (2.0)
-8.0 (-15.2, -0.8)
Placebo
45.3 (9.2)
1.7 (2.1)
--
Study 2
Quetiapine (250 mg/day)
38.9 (9.8)
-4.2 (1.6)
-3.2 (-7.6, 1.2)
Quetiapine (750 mg/day) 4
41.0 (9.6)
-8.7 (1.6)
-7.8 (-12.2, -3.4)
Placebo
38.4 (9.7)
-1.0 (1.6)
--
Study 3
Quetiapine (450 mg/day BID)
42.1 (10.7)
-10.0 (1.3)
-4.6 (-7.8, -1.4)
Quetiapine (450 mg/day TID) 5
42.7 (10.4)
-8.6 (1.3)
-3.2 (-6.4, 0.0)
Quetiapine (50 mg BID)
41.7 (10.0)
-5.4 (1.3)
--
Primary Efficacy Endpoint: PANSS Total
Mean Baseline
Score (SD)
LS Mean Change
from Baseline (SE)
Placebo-subtracted
Difference6 (95% CI)
Study 4
Quetiapine (400 mg/day) 4
96.2 (17.7)
-27.3 (2.6)
-8.2 (-16.1, -0.3)
Quetiapine (800 mg/day) 4
96.9 (15.3)
-28.4 (1.8)
-9.3 (-16.2, -2.4)
Placebo
96.2 (17.7)
-19.2 (3.0)
--
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval.
4. Doses that are statistically significant superior to placebo.
5. Doses that are statistically significant superior to quetiapine 50 mg BID.
6. Difference (drug minus placebo) in least-squares mean change from baseline.
14.2 Bipolar Disorder
Bipolar I disorder, manic or mixed episodes Adults The efficacy of quetiapine in the acute treatment of manic episodes was established in 3 placebo-controlled trials in patients who met DSM-IV criteria for bipolar I disorder with manic episodes. These trials included patients with or without psychotic features and excluded patients with rapid cycling and mixed episodes. Of these trials, 2 were monotherapy (12 weeks) and 1 was adjunct therapy (3 weeks) to either lithium or divalproex. Key outcomes in these trials were change from baseline in the Young Mania Rating Scale (YMRS) score at 3 and 12 weeks for monotherapy and at 3 weeks for adjunct therapy. Adjunct therapy is defined as the simultaneous initiation or subsequent administration of quetiapine with lithium or divalproex.
The primary rating instrument used for assessing manic symptoms in these trials was YMRS, an 11-item clinician-rated scale traditionally used to assess the degree of manic symptomatology (irritability, disruptive/aggressive behavior, sleep, elevated mood, speech, increased activity, sexual interest, language/thought disorder, thought content, appearance, and insight) in a range from 0 (no manic features) to 60 (maximum score).
The results of the trials follow:
Monotherapy
The efficacy of quetiapine in the acute treatment of bipolar mania was established in 2 placebo-controlled trials. In two 12-week trials (n=300, n=299) comparing quetiapine to placebo, quetiapine was superior to placebo in the reduction of the YMRS total score at weeks 3 and 12. The majority of patients in these trials taking quetiapine were dosed in a range between 400 mg/day and 800 mg per day (studies 1 and 2 in Table 20).
Adjunct Therapy
In this 3-week placebo-controlled trial, 170 patients with bipolar mania (YMRS ≥ 20) were randomized to receive quetiapine or placebo as adjunct treatment to lithium or divalproex. Patients may or may not have received an adequate treatment course of lithium or divalproex prior to randomization. Quetiapine was superior to placebo when added to lithium or divalproex alone in the reduction of YMRS total score. (study 3 in Table 20).
The majority of patients in this trial taking quetiapine were dosed in a range between 400 mg/day and 800 mg per day. In a similarly designed trial (n=200), quetiapine was associated with an improvement in YMRS scores but did not demonstrate superiority to placebo, possibly due to a higher placebo effect.
The primary efficacy results of these studies in the treatment of mania in adults is presented in Table 20. Children and Adolescents (ages 10-17) The efficacy of quetiapine in the acute treatment of manic episodes associated with bipolar I disorder in children and adolescents (10 to 17 years of age) was demonstrated in a 3-week, double-blind, placebo-controlled, multicenter trial (study 4 in Table 20). Patients who met DSM-IV diagnostic criteria for a manic episode were randomized into one of three treatment groups: Quetiapine 400 mg/day (n = 95), Quetiapine 600 mg/day (n = 98), or placebo (n = 91). Study medication was initiated at 50 mg/day and on day 2 increased to 100 mg/day (divided doses given two or three times daily). Subsequently, the dose was titrated to a target dose of 400 mg/day or 600 mg/day using increments of 100 mg/day, given in divided doses two or three times daily. The primary efficacy variable was the mean change from baseline in total YMRS score.
Quetiapine 400 mg/day and 600 mg/day were superior to placebo in the reduction of YMRS total score (Table 20).
Table 20: Mania Trials
Study
Number
Treatment Group
Primary Efficacy Measure: YMRS Total
Mean Baseline Score (SD)4
LS Mean Change from Baseline (SE)
Placebo-subtracted Difference2 (95% CI)
Study 1
Quetiapine (200-800 mg/day) 1, 3
34.0 (6.1)
-12.3 (1.3)
-4.0 (-7.0, -1.0)
Haloperidol 1, 3
32.3 (6.0)
-15.7 (1.3)
-7.4 (-10.4, -4.4)
Placebo
33.1 (6.6)
-8.3 (1.3)
--
Study 2
Quetiapine (200-800 mg/day) 1
32.7 (6.5)
-14.6 (1.5)
-7.9 (-10.9, -5.0)
Lithium 1, 3
33.3 (7.1)
-15.2 (1.6)
-8.5 (-11.5, -5.5)
Placebo
34.0 (6.9)
-6.7 (1.6)
--
Study 3
Quetiapine (200-800 mg/day) 1 + mood stabilizer
31.5 (5.8)
-13.8 (1.6)
-3.8 (-7.1, -0.6)
Placebo + mood stabilizer
31.1 (5.5)
-10 (1.5)
--
Study 4
Quetiapine (400 mg/day) 1
29.4 (5.9)
-14.3 (0.96)
-5.2 (-8.1, -2.3)
Quetiapine (600 mg/day) 1
29.6 (6.4)
-15.6 (0.97)
-6.6 (-9.5, -3.7)
Placebo
30.7 (5.9)
-9.0 (1.1)
--
Mood stabilizer: lithium or divalproex; SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval.
1. Doses that are statistically significantly superior to placebo.
2. Difference (drug minus placebo) in least-squares mean change from baseline.
3. Included in the trial as an active comparator.
4. Adult data mean baseline score is based on patients included in the primary analysis; pediatric mean baseline score is based on all patients in the ITT population.
Bipolar Disorder, Depressive Episodes
Adults
The efficacy of quetiapine for the acute treatment of depressive episodes associated with bipolar disorder was established in 2 identically designed 8-week, randomized, double-blind, placebo-controlled studies (N=1045) (studies 5 and 6 in Table 21). These studies included patients with either bipolar I or II disorder and those with or without a rapid cycling course. Patients randomized to quetiapine were administered fixed doses of either 300 mg or 600 mg once daily.
The primary rating instrument used to assess depressive symptoms in these studies was the Montgomery-Asberg Depression Rating Scale (MADRS), a 10-item clinician-rated scale with scores ranging from 0 to 60. The primary endpoint in both studies was the change from baseline in MADRS score at week 8. In both studies, quetiapine was superior to placebo in reduction of MADRS score. Improvement in symptoms, as measured by change in MADRS score relative to placebo, was seen in both studies at Day 8 (week 1) and onwards. In these studies, no additional benefit was seen with the 600 mg dose. For the 300 mg dose group, statistically significant improvements over placebo were seen in overall quality of life and satisfaction related to various areas of functioning, as measured using the Q-LES-Q(SF).
The primary efficacy results of these studies in the acute treatment of depressive episodes associated with bipolar disorder in adults is presented in Table 21.
Table 21: Depressive Episodes Associated with Bipolar Disorder
Study
Number
Treatment Group
Primary Efficacy Measure: MADRS Total
Mean Baseline Score (SD)
LS Mean Change
from Baseline (SE)
Placebo-subtracted Difference2 (95% CI)
Study 5
Quetiapine (300 mg/day) 1
30.3 (5.0)
-16.4 (0.9)
-6.1 (-8.3, -3.9)
Quetiapine (600 mg/day) 1
30.3 (5.3)
-16.7 (0.9)
-6.5 (-8.7, -4.3)
Placebo
30.6 (5.3)
-10.3 (0.9)
--
Study 6
Quetiapine (300 mg/day) 1
31.1 (5.7)
-16.9 (1.0)
-5.0 (-7.3, -2.7)
Quetiapine (600 mg/day) 1
29.9 (5.6)
-16.0 (1.0)
-4.1 (-6.4, -1.8)
Placebo
29.6 (5.4)
-11.9 (1.0)
--
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: unadjusted confidence interval.
1. Doses that are statistically significantly superior to placebo.
2. Difference (drug minus placebo) in least-squares mean change from baseline.
Maintenance Treatment as an Adjunct to Lithium or Divalproex
The efficacy of quetiapine in the maintenance treatment of bipolar I disorder was established in 2 placebo-controlled trials in patients (n=1326) who met DSM-IV criteria for bipolar I disorder (studies 7 and 8 in Figures 1 and 2). The trials included patients whose most recent episode was manic, depressed, or mixed, with or without psychotic features. In the open-label phase, patients were required to be stable on quetiapine plus lithium or divalproex for at least 12 weeks in order to be randomized. On average, patients were stabilized for 15 weeks. In the randomization phase, patients continued treatment with lithium or divalproex and were randomized to receive either quetiapine (administered twice daily totaling 400 mg/day to 800 mg/day) or placebo. Approximately 50% of the patients had discontinued from the quetiapine group by day 280 and 50% of the placebo group had discontinued by day 117 of double-blind treatment. The primary endpoint in these studies was time to recurrence of a mood event (manic, mixed or depressed episode). A mood event was defined as medication initiation or hospitalization for a mood episode; YMRS score ≥ 20 or MADRS score ≥ 20 at 2 consecutive assessments; or study discontinuation due to a mood event. (Figure 1 and Figure 2).
In both studies, quetiapine was superior to placebo in increasing the time to recurrence of any mood event. The treatment effect was present for increasing time to recurrence of both manic and depressed episodes. The effect of quetiapine was independent of any specific subgroup (assigned mood stabilizer, sex, age, race, most recent bipolar episode, or rapid cycling course).
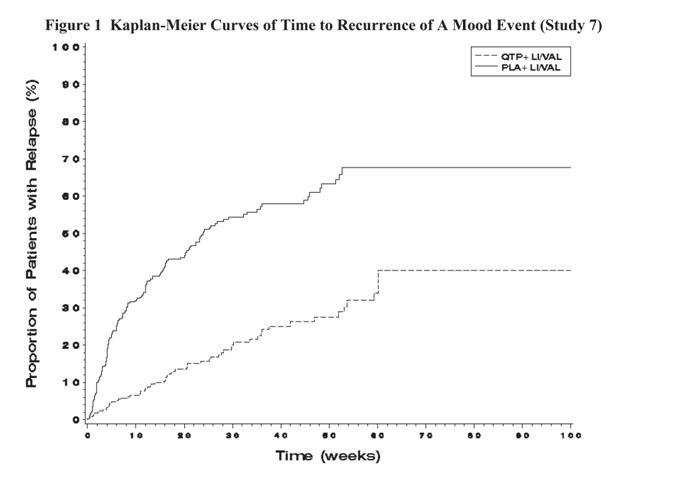
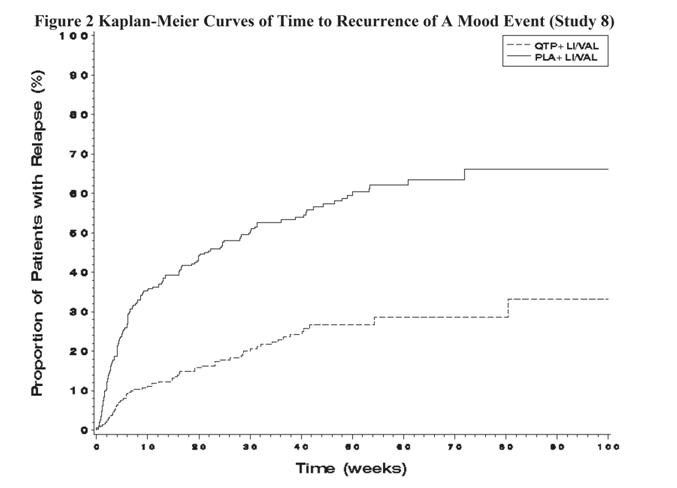
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Quetiapine tablets, USP 25 mg
Peach coloured, film coated, round shape, biconvex tablets, debossed with "262 ” on one side and plain on other side.
Bottles of 30 tablets NDC: 68071-1892-3
Store at 25°C (77°F); excursions permitted to 15°C to 30°C (59° to 86°F) [See USP].
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Medication Guide)
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with quetiapine and should counsel them in its appropriate use. A patient Medication Guide about “Antidepressant Medicines, Depression and other Serious Mental Illness, and Suicidal Thoughts or Actions” is available for quetiapine. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document.
Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking quetiapine.
Increased Mortality in Elderly Patients with Dementia-Related Psychosis Patients and caregivers should be advised that elderly patients with dementia-related psychosis treated with atypical antipsychotic drugs are at increased risk of death compared with placebo. Quetiapine is not approved for elderly patients with dementia-related psychosis [see Warnings and Precautions (5.1)] .
Suicidal Thoughts and Behaviors
Patients, their families, and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, mania, other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down. Families and caregivers of patients should be advised to look for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt. Such symptoms should be reported to the patient's prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patient's presenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behavior and indicate a need for very close monitoring and possibly changes in the medication [see Warnings and Precautions (5.2)].
Neuroleptic Malignant Syndrome (NMS)
Patients should be advised to report to their physician any signs or symptoms that may be related to NMS. These may include muscle stiffness and high fever [see Warnings and Precautions (5.4)].
Hyperglycemia and Diabetes Mellitus
Patients should be aware of the symptoms of hyperglycemia (high blood sugar) and diabetes mellitus. Patients who are diagnosed with diabetes, those with risk factors for diabetes, or those that develop these symptoms during treatment should have their blood glucose monitored at the beginning of and periodically during treatment [see Warnings and Precautions (5.5)].
Hyperlipidemia
Patients should be advised that elevations in total cholesterol, LDL-cholesterol and triglycerides and decreases in HDL- cholesterol may occur. Patients should have their lipid profile monitored at the beginning of and periodically during treatment [see Warnings and Precautions (5.5)] .
Weight Gain
Patients should be advised that they may experience weight gain. Patients should have their weight monitored regularly [see Warnings and Precautions (5.5)].
Orthostatic Hypotension
Patients should be advised of the risk of orthostatic hypotension (symptoms include feeling dizzy or lightheaded upon standing, which may lead to falls), especially during the period of initial dose titration, and also at times of re-initiating treatment or increases in dose [see Warnings and Precautions (5.7)] .
Increased Blood Pressure in Children and Adolescents
Children and adolescent patients should have their blood pressure measured at the beginning of, and periodically during, treatment [ see Warnings and Precautions (5.8) ].
Leukopenia/Neutropenia
Patients with a pre-existing low WBC or a history of drug induced leukopenia/neutropenia should be advised that they should have their CBC monitored while taking quetiapine [ see Warnings and Precautions (5.9) ].
Interference with Cognitive and Motor Performance
Patients should be advised of the risk of somnolence or sedation (which may lead to falls), especially during the period of initial dose titration. Patients should be cautioned about performing any activity requiring mental alertness, such as operating a motor vehicle (including automobiles) or operating machinery, until they are reasonably certain quetiapine therapy does not affect them adversely. [ see Warnings and Precautions (5.15) ].
Heat Exposure and Dehydration
Patients should be advised regarding appropriate care in avoiding overheating and dehydration [ see Warnings and Precautions (5.16) ].
Concomitant Medication
As with other medications, patients should be advised to notify their physicians if they are taking, or plan to take, any prescription or over-the-counter drugs. [ see Drug Interactions (7.1)].
Pregnancy and Nursing
Patients should be advised to notify their physician if they become pregnant or intend to become pregnant during therapy with quetiapine. [ see Use in Specific Populations (8.1) and (8.3)].
Need for Comprehensive Treatment Program
Quetiapine is indicated as an integral part of a total treatment program for adolescents with schizophrenia and pediatric bipolar disorder that may include other measures (psychological, educational, and social). Effectiveness and safety of quetiapine have not been established in pediatric patients less than 13 years of age for schizophrenia or less than 10 years of age for bipolar mania. Appropriate educational placement is essential and psychosocial intervention is often helpful. The decision to prescribe atypical antipsychotic medication will depend upon the physician’s assessment of the chronicity and severity of the patient’s symptoms [ see Indications and Usage (1.3)].
PT 1438-06
Medication Guide
Quetiapine Tablets
(kwe-TYE-a-peen)
Read this Medication Guide before you start taking quetiapine tablets and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about quetiapine?
Quetiapine may cause serious side effects, including:
1. risk of death in the elderly with dementia. Medicines like quetiapine can increase the risk of death in elderly people who have memory loss (dementia). Quetiapine is not for treating psychosis in the elderly with dementia.
2. risk of suicidal thoughts or actions (antidepressant medicines, depression and other serious mental illnesses, and suicidal thoughts or actions).
- Talk to your or your family member’s healthcare provider about:
- all risks and benefits of treatment with antidepressant medicines.
- all treatment choices for depression or other serious mental illness
- Antidepressant medications may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
- Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions. These include people who have (or have a family history of) depression, bipolar illness (also called manic-depressive illness), or suicidal thoughts or actions.
- How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
- Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
- Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
- Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
Call a healthcare provider right away if you or your family member has any of the following symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling very agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
What else do I need to know about antidepressant medicines?
- Never stop an antidepressant medicine without first talking to your healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
- Antidepressants are medicines used to treat depression and other illnesses. It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
- Antidepressant medicines have other side effects. Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
- Antidepressant medicines can interact with other medicines. Know all of the medicines that you or your family member take. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
- Not all antidepressant medicines prescribed for children are FDA approved for use in children. Talk to your child’s healthcare provider for more information.
What is quetiapine?
Quetiapine is a prescription medicine used to treat:
- schizophrenia in people 13 years of age or older
- bipolar disorder in adults, including:
- depressive episodes associated with bipolar disorder
- manic episodes associated with bipolar I disorder alone or with lithium or divalproex
- long-term treatment of bipolar I disorder with lithium or divalproex
- manic episodes associated with bipolar I disorder in children ages 10 to 17 years old.
It is not known if quetiapine is safe and effective in children under 10 years of age. What should I tell my healthcare provider before taking quetiapine?
Before you take quetiapine, tell your healthcare provider if you have or have had:
- diabetes or high blood sugar in you or your family. Your healthcare provider should check your blood sugar before you start quetiapine and also during therapy
- high levels of total cholesterol, triglycerides or LDL-cholesterol or low levels of HDL-cholesterol
- low or high blood pressure x low white blood cell count x cataracts
- seizures
- abnormal thyroid tests
- high prolactin levels
- heart problems
- liver problems
- any other medical condition
- pregnancy or plans to become pregnant. It is not known if quetiapine will harm your unborn baby.
- Breast-feeding or plans to breast-feed. Quetiapine can pass into your breast milk. You and your healthcare provider should decide if you will take quetiapine or breast-feed. You should not do both.
Tell the healthcare provider about all the medicines that you take or recently have taken including prescription medicines, over-the-counter medicines, herbal supplements and vitamins.
Quetiapine and other medicines may affect each other causing serious side effects. Quetiapine may affect the way other medicines work, and other medicines may affect how quetiapine works.
Tell your healthcare provider if you are having a urine drug screen because quetiapine may affect your test results. Tell those giving the test that you are taking quetiapine.
How should I take quetiapine?
- Take quetiapine exactly as your healthcare provider tells you to take it. Do not change the dose yourself.
- Take quetiapine by mouth, with or without food.
- If you feel you need to stop quetiapine, talk with your healthcare provider first. If you suddenly stop taking quetiapine, you may have side effects such as trouble sleeping or trouble staying asleep (insomnia), nausea, and vomiting.
- If you miss a dose of quetiapine, take it as soon as you remember. If you are close to your next dose, skip the missed dose. Just take the next dose at your regular time. Do not take 2 doses at the same time unless your healthcare provider tells you to. If you are not sure about your dosing, call your healthcare provider.
What should I avoid while taking quetiapine?
- Do not drive, operate machinery, or do other dangerous activities until you know how quetiapine affects you. Quetiapinemay make you drowsy.
- Avoid getting overheated or dehydrated.
- Do not over-exercise.
- In hot weather, stay inside in a cool place if possible.
- Stay out of the sun. Do not wear too much or heavy clothing.
- Drink plenty of water.
- Do not drink alcohol while taking quetiapine. It may make some side effects of quetiapine worse.
What are possible side effects of quetiapine?
Quetiapine can cause serious side effects, including:
- See “What is the most important information I should know about quetiapine?”
- stroke that can lead to death can happen in elderly people with dementia who take medicines like quetiapine
- neuroleptic malignant syndrome (NMS). NMS is a rare but very serious condition that can happen in people who take antipsychotic medicines, including quetiapine. NMS can cause death and must be treated in a hospital. Call your healthcare provider right away if you become severely ill and have some or all of these symptoms:
- high fever
- excessive sweating
- rigid muscles
- confusion
- changes in your breathing, heartbeat, and blood pressure
-
high blood sugar (hyperglycemia). High blood sugar can happen if you have diabetes already or if you have never had diabetes. High blood sugar could lead to:
- build up of acid in your blood due to ketones (ketoacidosis)
- coma
- death
Increases in blood sugar can happen in some people who take quetiapine. Extremely high blood sugar can lead to coma or death. If you have diabetes or risk factors for diabetes (such as being overweight or a family history of diabetes) your healthcare provider should check your blood sugar before you start quetiapine and during therapy. Call your healthcare provider if you have any of these symptoms of high blood sugar (hyperglycemia) while taking quetiapine:
- feel very thirsty
- need to urinate more than usual
- feel very hungry
- feel weak or tired
- feel sick to your stomach
- feel confused, or your breath smells fruity
- high fat levels in your blood (increased cholesterol and triglycerides). High fat levels may happen in people treated with quetiapine. You may not have any symptoms, so your healthcare provider may decide to check your cholesterol and triglycerides during your treatment with quetiapine.
- increase in weight (weight gain). Weight gain is common in people who take quetiapine so you and your healthcare provider should check your weight regularly. Talk to your healthcare provider about ways to control weight gain, such as eating a healthy, balanced diet, and exercising.
- movements you cannot control in your face, tongue, or other body parts (tardive dyskinesia). These may be signs of a serious condition. Tardive dyskinesia may not go away, even if you stop taking quetiapine. Tardive dyskinesia may also start after you stop taking quetiapine.
- decreased blood pressure (orthostatic hypotension), including lightheadedness or fainting caused by a sudden change in heart rate and blood pressure when rising too quickly from a sitting or lying position.
- increases in blood pressure in children and teenagers. Your healthcare provider should check blood pressure in children and adolescents before starting quetiapine and during therapy.
- low white blood cell count
- cataracts
- seizures
- abnormal thyroid tests. Your healthcare provider may do blood tests to check your thyroid hormone level.
- increases in prolactin levels. Your healthcare provider may do blood tests to check your prolactin levels.
- sleepiness, drowsiness, feeling tired, difficulty thinking and doing normal activities
- increased body temperature
- difficulty swallowing
- trouble sleeping or trouble staying asleep (insomnia), nausea, or vomiting if you suddenly stop taking quetiapine. These symptoms usually get better 1 week after you start having them.
The most common side effects of quetiapine include:
In Adults:
- dry mouth
- dizziness
- weakness
- abdominal pain
- constipation
- sore throat
- difficulty moving
In Children and Adolescents:
- Nausea
- Dry mouth
- Weight gain
- Increase appetite
- Vomiting
- Rapid heart beat
These are not all the possible side effects of quetiapine. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store quetiapine?
- Store quetiapine at room temperature, between 68°F to 77°F (20°C to 25°C).
- Keep quetiapine and all medicines out of the reach of children.
General information about the safe and effective use of quetiapine.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use quetiapinefor a condition for which it was not prescribed. Do not give quetiapine to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about quetiapine. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about quetiapine that is written for health professionals.
What are the ingredients in quetiapine? Active ingredient: quetiapine fumarate
Inactive ingredients: povidone, dibasic dicalcium phosphate dihydrate, microcrystalline cellulose, sodium starch glycolate, lactose monohydrate, magnesium stearate, hypromellose, polyethylene glycol and titanium dioxide.The 25 mg tablets contain red iron oxide and yellow iron oxide and the 100 mg, 150 mg and 400 mg tablets contain only yellow iron oxide.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured in India by:
Alkem Laboratories Limited
Mumbai – 400 013, INDIA
Distributed by:
Ascend Laboratories, LLC
Montvale, NJ07645
Revised: October 2015
PT 1439-02 - PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
QUETIAPINE FUMARATE
quetiapine fumarate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68071-1892(NDC:67877-242) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength QUETIAPINE FUMARATE (UNII: 2S3PL1B6UJ) (QUETIAPINE - UNII:BGL0JSY5SI) QUETIAPINE 25 mg Inactive Ingredients Ingredient Name Strength DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) POVIDONE K30 (UNII: U725QWY32X) MAGNESIUM STEARATE (UNII: 70097M6I30) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) POLYETHYLENE GLYCOL 4000 (UNII: 4R4HFI6D95) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color pink (peach) Score no score Shape ROUND (biconvex) Size 6mm Flavor Imprint Code 262 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68071-1892-3 30 in 1 BOTTLE; Type 0: Not a Combination Product 11/10/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA201504 03/01/2013 Labeler - NuCare Pharmaceuticals, Inc. (010632300) Establishment Name Address ID/FEI Business Operations NuCare Pharmaceuticals, Inc. 010632300 repack(68071-1892)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
