REVLON COLORSTAY LIQUID MAKEUP FOR OILY TO COMBINATION SKIN- zinc oxide, titanium dioxide make-up liquid
Revlon Colorstay Liquid Makeup for Oily to Combination Skin by
Drug Labeling and Warnings
Revlon Colorstay Liquid Makeup for Oily to Combination Skin by is a Otc medication manufactured, distributed, or labeled by Revlon Consumer Products Corp, REVLON, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredients:
- Uses:
-
Warnings:
- For external use only
- Do notuse on damaged or broken skin
- When using this product: Keep out of eyes. Rinse with water to remove.
- Stop use and ask a doctor if a rash occurs
- Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control center right away
Directions:
- Apply liberally 15 minutes before sun exposure
- Use a water resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours
- Children under 6 months: Ask a doctor.
Sun Protection Measures:
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 am - 2 pm
- Wear long-sleeved shirts, pants, hats and sunglasses.
-
Inactive Ingredients:
CYCLOPENTASILOXANE,AQUA ((WATER) EAU),DIMETHICONE,TRIMETHYLSILOXYSILICATE,PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE,BUTYLENE GLYCOL,TRIBEHENIN,PHENYL TRIMETHICONE,NYLON-12,ALCOHOL DENAT.,ALUMINA,DIMETHICONE/PEG-10/15 CROSSPOLYMER,METHICONE,LAURETH-7,MAGNESIUM SULFATE,SILICA,POLYGLYCERYL-3 DIISOSTEARATE,PHENOXYETHANOL,ETHYLPARABEN,METHYLPARABEN,ETHYLENE BRASSYLATE,TRIETHOXYCAPRYLYLSILANE,TOCOPHERYL ACETATE,TETRASODIUM EDTA,DIMETHICONE/SILSESQUIOXANE COPOLYMER,BISABOLOL,SILK POWDER,ETHYLHEXYL PALMITATE,TOCOPHEROL,SODIUM CITRATE,DIPROPYLENE GLYCOL ,SALICYLIC ACID,SILICA DIMETHICONE SILYLATE,MALVIA SYLVESTRIS (MALLOW) EXTRACT,LILIUM CANDIDUM BULB EXTRACT,LACTOBACILLUS/ERIODICTYON CALIFORNICUM FERMENT EXTRACT,CYMBIDIUM GRADIFLORUM (ORCHID) FLOWER EXTRACT,SODIUM HYALURONATE,SERICA ((SILK) SOIE),HEXYLENE GLYCOL,CAPRYLYL GLYCOL,
May Contain Mica, Titanium Dioxide (Ci 77891), Iron Oxides (CI 77491, 77492, 77499)
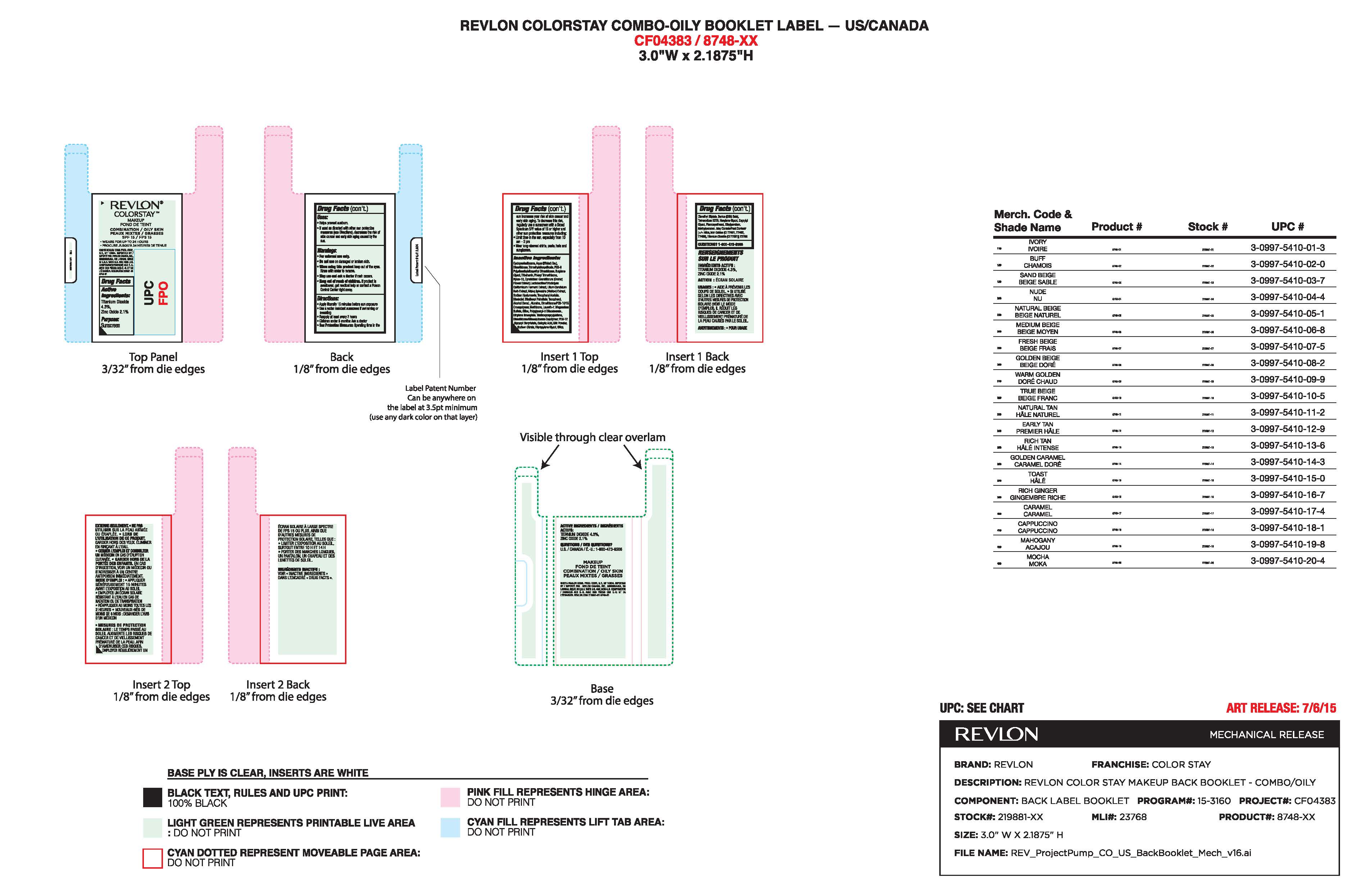
- Principal Display Panel:
- Package Label for Revlon Colorstay Liquid Makeup for Oily to Combination Skin
-
INGREDIENTS AND APPEARANCE
REVLON COLORSTAY LIQUID MAKEUP FOR OILY TO COMBINATION SKIN
zinc oxide, titanium dioxide make-up liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 10967-642 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 2.1 mg in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 4.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) METHICONE (20 CST) (UNII: 6777U11MKT) LAURETH-7 (UNII: Z95S6G8201) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) ETHYLPARABEN (UNII: 14255EXE39) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: TYP81E471F) TRIBEHENIN (UNII: 8OC9U7TQZ0) NYLON-12 (UNII: 446U8J075B) ALCOHOL (UNII: 3K9958V90M) ALUMINUM OXIDE (UNII: LMI26O6933) MAGNESIUM SULFATE, UNSPECIFIED FORM (UNII: DE08037SAB) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYALURONATE SODIUM (UNII: YSE9PPT4TH) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) ETHYLENE BRASSYLATE (UNII: 9A87HC7ROD) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) EDETATE SODIUM (UNII: MP1J8420LU) LEVOMENOL (UNII: 24WE03BX2T) ETHYLHEXYL PALMITATE (UNII: 2865993309) TOCOPHEROL (UNII: R0ZB2556P8) SODIUM CITRATE (UNII: 1Q73Q2JULR) DIPROPYLENE GLYCOL (UNII: E107L85C40) SALICYLIC ACID (UNII: O414PZ4LPZ) MALVA SYLVESTRIS FLOWERING TOP (UNII: X1U1U0N90J) LILIUM CANDIDUM BULB (UNII: AHG15J8AM0) CYMBIDIUM HOOKERIANUM FLOWER (UNII: H2DJ03DI5I) HEXYLENE GLYCOL (UNII: KEH0A3F75J) CAPRYLYL GLYCOL (UNII: 00YIU5438U) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 10967-642-01 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 10/27/2015 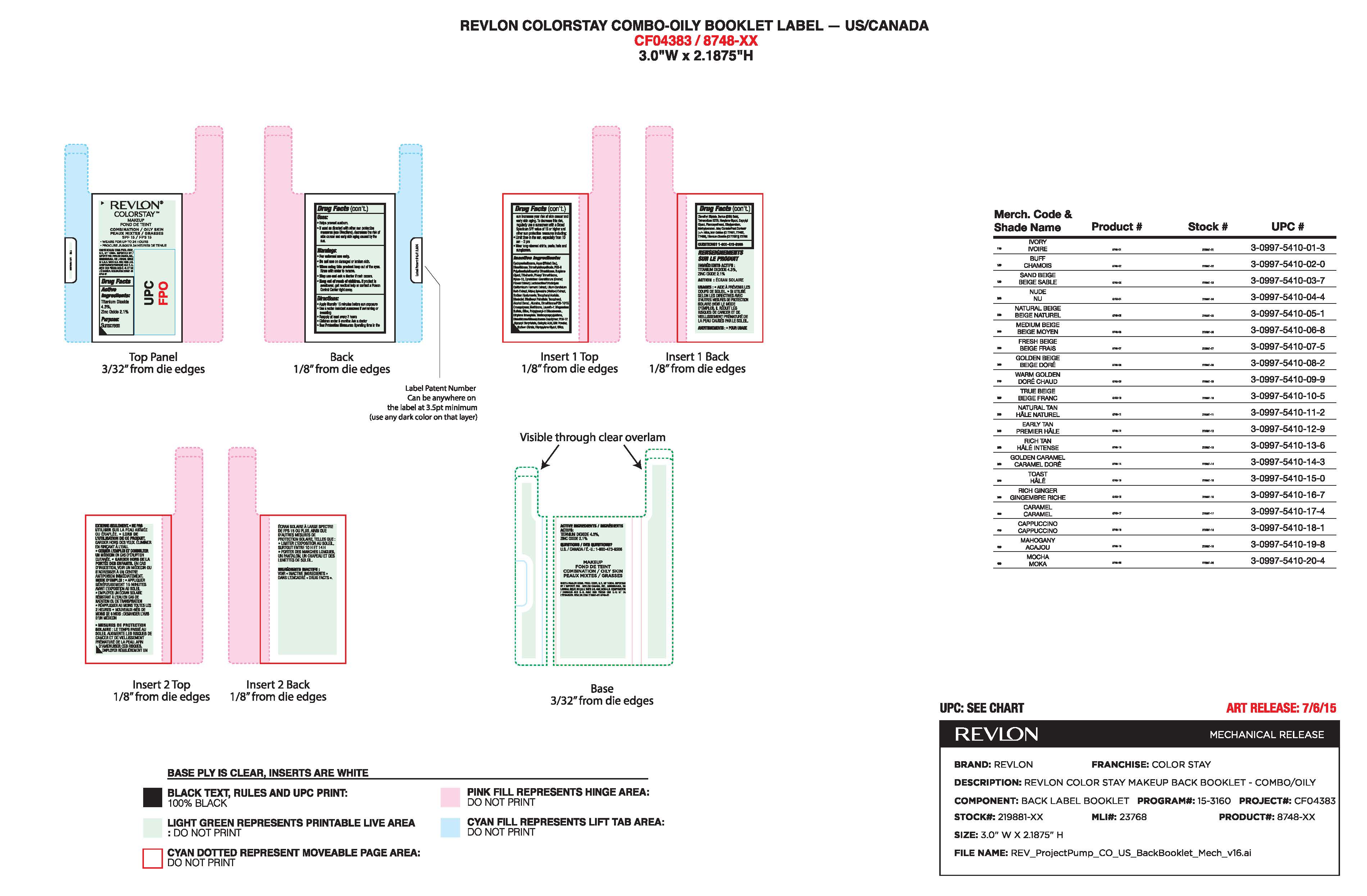
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 10/27/2014 Labeler - Revlon Consumer Products Corp (788820165) Establishment Name Address ID/FEI Business Operations Kolmar Laboratories, Inc. 001535103 manufacture(10967-642) Establishment Name Address ID/FEI Business Operations Dermaceutical Laboratories Limited Liability Company 078457159 manufacture(10967-642) Establishment Name Address ID/FEI Business Operations REVLON, INC. 809725570 manufacture(10967-642)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.