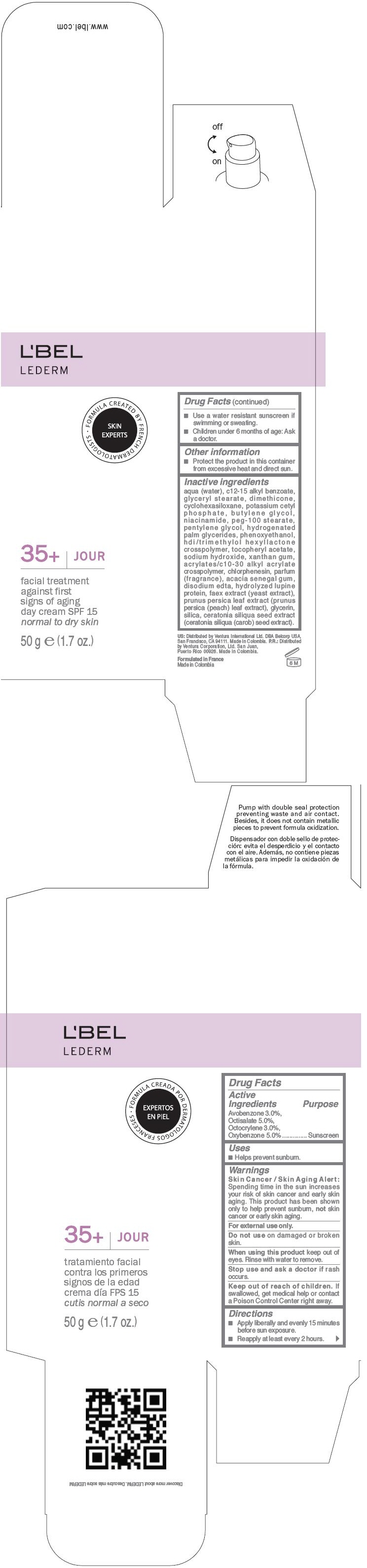
LBEL LEDERM 35PLUS JOUR SPF 15 FACIAL TREATMENT AGAINST FIRST SIGNS OF AGING DAY - NORMAL TO DRY SKIN- avobenzone, octisalate, octocrylene, and oxybenzone cream
LBEL LEDERM 35plus Jour by
Drug Labeling and Warnings
LBEL LEDERM 35plus Jour by is a Otc medication manufactured, distributed, or labeled by Ventura Corporation LTD., Bel Star S.A. (Colombia), Yobel Supply Chain Management S.A. (Peru). Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
- Directions
- Other information
-
Inactive ingredients
AQUA (WATER), C12-15 ALKYL BENZOATE, GLYCERYL STEARATE, DIMETHICONE, CYCLOHEXASILOXANE, POTASSIUM CETYL PHOSPHATE, BUTYLENE GLYCOL, NIACINAMIDE, PEG-100 STEARATE, PENTYLENE GLYCOL, HYDROGENATED PALM GLYCERIDES, PHENOXYETHANOL, HDI/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER, TOCOPHERYL ACETATE, SODIUM HYDROXIDE, XANTHAN GUM, ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER, CHLORPHENESIN, PARFUM (FRAGRANCE), ACACIA SENEGAL GUM, DISODIUM EDTA, HYDROLYZED LUPINE PROTEIN, FAEX EXTRACT (YEAST EXTRACT), PRUNUS PERSICA LEAF EXTRACT (PRUNUS PERSICA (PEACH) LEAF EXTRACT), GLYCERIN, SILICA, CERATONIA SILIQUA SEED EXTRACT (CERATONIA SILIQUA (CAROB) SEED EXTRACT)
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 50 g Bottle Carton
-
INGREDIENTS AND APPEARANCE
LBEL LEDERM 35PLUS JOUR SPF 15 FACIAL TREATMENT AGAINST FIRST SIGNS OF AGING DAY - NORMAL TO DRY SKIN
avobenzone, octisalate, octocrylene, and oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 13537-445 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 0.03 g in 1 g Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 0.05 g in 1 g Octocrylene (UNII: 5A68WGF6WM) (Octocrylene - UNII:5A68WGF6WM) Octocrylene 0.03 g in 1 g Oxybenzone (UNII: 95OOS7VE0Y) (Oxybenzone - UNII:95OOS7VE0Y) Oxybenzone 0.05 g in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) DIMETHICONE (UNII: 92RU3N3Y1O) CYCLOMETHICONE 6 (UNII: XHK3U310BA) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) NIACINAMIDE (UNII: 25X51I8RD4) PEG-100 STEARATE (UNII: YD01N1999R) PENTYLENE GLYCOL (UNII: 50C1307PZG) HYDROGENATED PALM GLYCERIDES (UNII: YCZ8EM144Q) PHENOXYETHANOL (UNII: HIE492ZZ3T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SODIUM HYDROXIDE (UNII: 55X04QC32I) XANTHAN GUM (UNII: TTV12P4NEE) CHLORPHENESIN (UNII: I670DAL4SZ) ACACIA (UNII: 5C5403N26O) EDETATE DISODIUM (UNII: 7FLD91C86K) YEAST (UNII: 3NY3SM6B8U) PRUNUS PERSICA LEAF (UNII: VN3501T41P) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CAROB (UNII: 5MG5Z946UO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13537-445-01 1 g in 1 PACKET 2 NDC: 13537-445-04 1 in 1 BOX 2 NDC: 13537-445-03 5 g in 1 BOTTLE, PLASTIC 3 NDC: 13537-445-06 1 in 1 BOX 3 NDC: 13537-445-05 50 g in 1 BOTTLE, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 04/01/2013 Labeler - Ventura Corporation LTD. (602751344) Establishment Name Address ID/FEI Business Operations Bel Star S.A. (Colombia) 880160197 MANUFACTURE(13537-445) Establishment Name Address ID/FEI Business Operations Yobel Supply Chain Management S.A. (Peru) 934116930 MANUFACTURE(13537-445)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.