DRAMAMINE- dimenhydrinate tablet
Dramamine by
Drug Labeling and Warnings
Dramamine by is a Otc medication manufactured, distributed, or labeled by Navajo Manufacturing Company Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
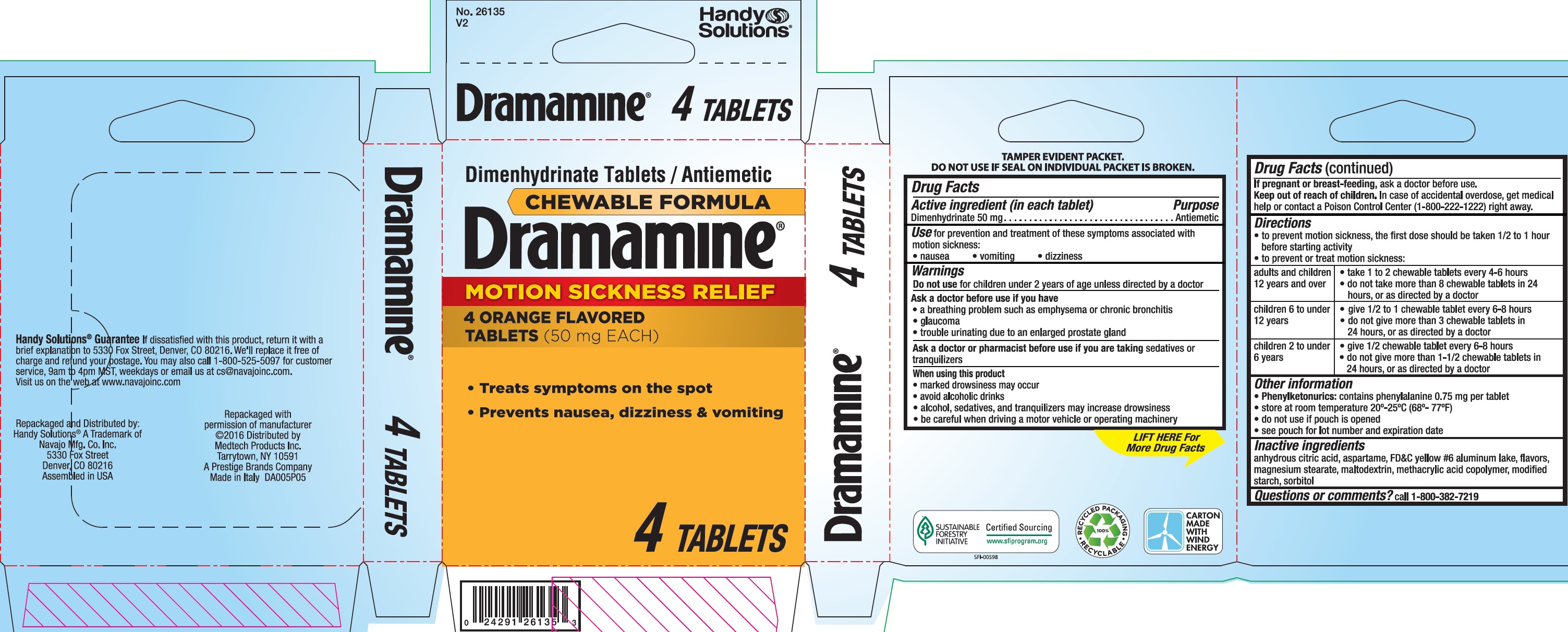
- Drug Facts
- Active ingredient (in each tablet)
- Use
-
Warnings
Ask a doctor before use if you have
a breathing problem such as emphysema or chronic bronchitis
glaucoma
trouble urinating due to an enlarged prostate gland -
Directions
to prevent motion sickness, the first dose should be taken 1/2 to 1 hour before starting activity
to prevent or treat motion sickness:adults and children 12 years and over take 1 to 2 chewable tablets every 4-6 hours
do not take more than 8 chewable tablets in 24 hours, or as directed by a doctor
children 6 to under 12 years give 1/2 to 1 chewable tablet every 6-8 hours
do not give more than 3 chewable tablets in 24 hours, or as directed by a doctor
children 2 to under 6 years give 1/2 chewable tablet every 6-8 hours
do not give more than 1-1/2 chewable tablets in 24 hours, or as directed by a doctor
- Other information
- Inactive ingredients
- Questions or comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
DRAMAMINE
dimenhydrinate tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 67751-170(NDC:63029-901) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMENHYDRINATE (UNII: JB937PER5C) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIMENHYDRINATE 50 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) ASPARTAME (UNII: Z0H242BBR1) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) SORBITOL (UNII: 506T60A25R) Product Characteristics Color orange Score 2 pieces Shape ROUND Size 8mm Flavor ORANGE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67751-170-01 1 in 1 CARTON 09/23/2016 1 2 in 1 POUCH; Type 0: Not a Combination Product 2 NDC: 67751-170-02 1 in 1 CARTON 09/23/2016 2 4 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part336 09/23/2016 Labeler - Navajo Manufacturing Company Inc. (091917799) Establishment Name Address ID/FEI Business Operations Navajo Manufacturing Company Inc. 136941411 relabel(67751-170) , repack(67751-170)
Trademark Results [Dramamine]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DRAMAMINE 86112919 4551701 Live/Registered |
Medtech Products Inc. 2013-11-07 |
 DRAMAMINE 71575604 0527862 Live/Registered |
G.D. SEARLE & CO. 1949-03-17 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.