VYNDAQEL- tafamidis meglumine capsule
Vyndaqel by
Drug Labeling and Warnings
Vyndaqel by is a Prescription medication manufactured, distributed, or labeled by Pfizer Labs Division of Pfizer Inc., DELMAR CHEMICALS INC, Catalent Pharma Solutions, LLC, AndersonBrecon Inc., Catalent Micron Technologies, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
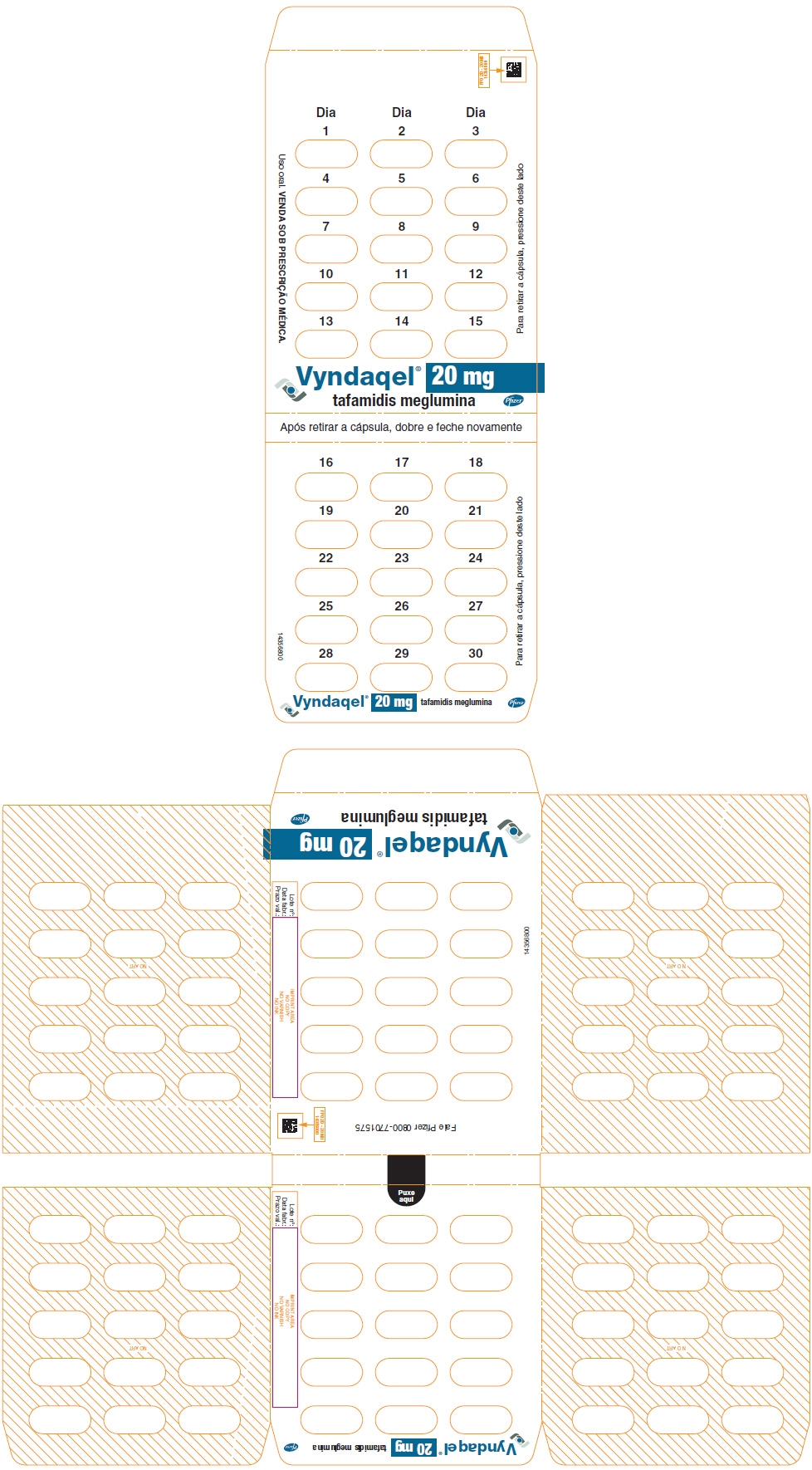
- PRINCIPAL DISPLAY PANEL - 20 mg Blister Pack Carton
- PRINCIPAL DISPLAY PANEL - 20 mg Blister Pack
-
INGREDIENTS AND APPEARANCE
VYNDAQEL
tafamidis meglumine capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0069-8729 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TAFAMIDIS MEGLUMINE (UNII: ZU7CF08A1A) (TAFAMIDIS - UNII:8FG9H9D31J) TAFAMIDIS MEGLUMINE 20 mg Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) SORBITOL (UNII: 506T60A25R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) POLYSORBATE 80 (UNII: 6OZP39ZG8H) Product Characteristics Color WHITE Score no score Shape CAPSULE Size 10mm Flavor Imprint Code VYN20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0069-8729-55 1 in 1 CARTON 12/15/2015 1 30 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date EXPORT ONLY 12/15/2015 Labeler - Pfizer Labs Division of Pfizer Inc. (134489525) Establishment Name Address ID/FEI Business Operations DELMAR CHEMICALS INC 245906755 API MANUFACTURE(0069-8729) Establishment Name Address ID/FEI Business Operations Catalent Pharma Solutions, LLC 051762268 ANALYSIS(0069-8729) , MANUFACTURE(0069-8729) Establishment Name Address ID/FEI Business Operations AndersonBrecon Inc. 053217022 LABEL(0069-8729) , PACK(0069-8729) Establishment Name Address ID/FEI Business Operations Catalent Micron Technologies, Inc. 015966157 PARTICLE SIZE REDUCTION(0069-8729)
Trademark Results [Vyndaqel]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VYNDAQEL 87706835 not registered Live/Pending |
Wyeth LLC 2017-12-04 |
 VYNDAQEL 77955739 4106105 Live/Registered |
Wyeth LLC 2010-03-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.