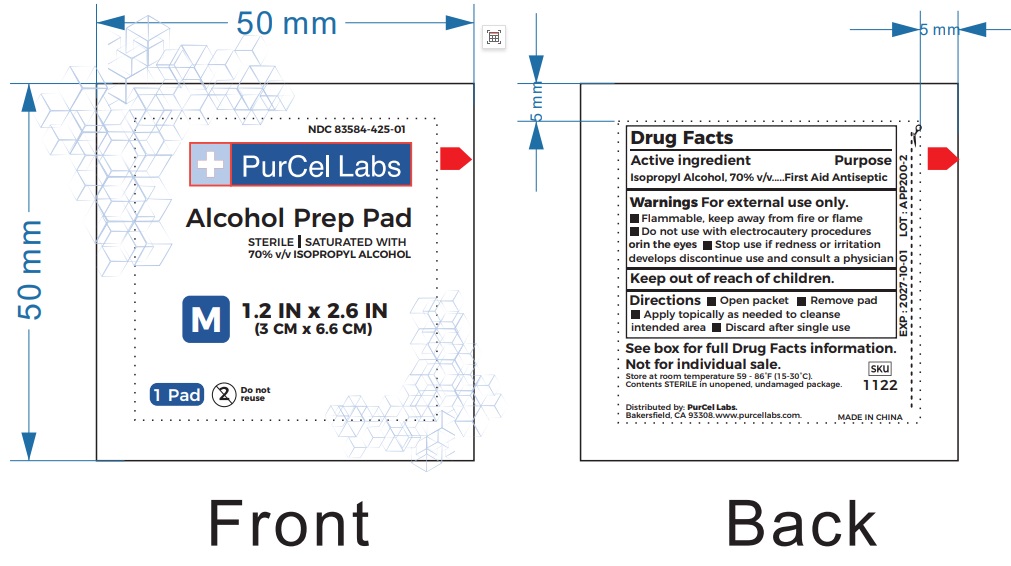
Alcohol Prep Pad by PurCel Labs LLC / JINHUA JINGDI MEDICAL SUPPLIES CO., LTD 83584-425
Alcohol Prep Pad by
Drug Labeling and Warnings
Alcohol Prep Pad by is a Otc medication manufactured, distributed, or labeled by PurCel Labs LLC, JINHUA JINGDI MEDICAL SUPPLIES CO., LTD. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ALCOHOL PREP PAD- 70% isopropy alcohol swab
PurCel Labs LLC
----------
83584-425
Warnings
For external use only.
Flammable,keep away from fire or flame
Do not use with electrocautery proceduresorin the eyes
Stop use if redness or irritationdevelops discontinue use and consult a physician
| ALCOHOL PREP PAD
70% isopropy alcohol swab |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - PurCel Labs LLC (127672531) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| JINHUA JINGDI MEDICAL SUPPLIES CO., LTD | 546604382 | manufacture(83584-425) | |
Revised: 12/2025
Document Id: 451b156c-90c6-546a-e063-6294a90ab6bf
Set id: 41827727-9461-d724-e063-6294a90a84ea
Version: 2
Effective Time: 20251204
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.