SUNMARK MUCUS RELIEF- guaifenesin tablet, extended release
Sunmark Mucus Relief by
Drug Labeling and Warnings
Sunmark Mucus Relief by is a Otc medication manufactured, distributed, or labeled by Strategic Sourcing Services LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT(in each extended-release tablet)
- PURPOSE
- USE(S)
- WARNING
- DO NOT USE
- ASK A DOCTOR BEFORE USE IF YOU HAVE
- STOP USE AND ASK A DOCTOR IF
- IF PREGNANT OR BREAST-FEEDING,
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
- OTHER INFORMATION
- INACTIVE INGREDIENTS
- QUESTIONS?
-
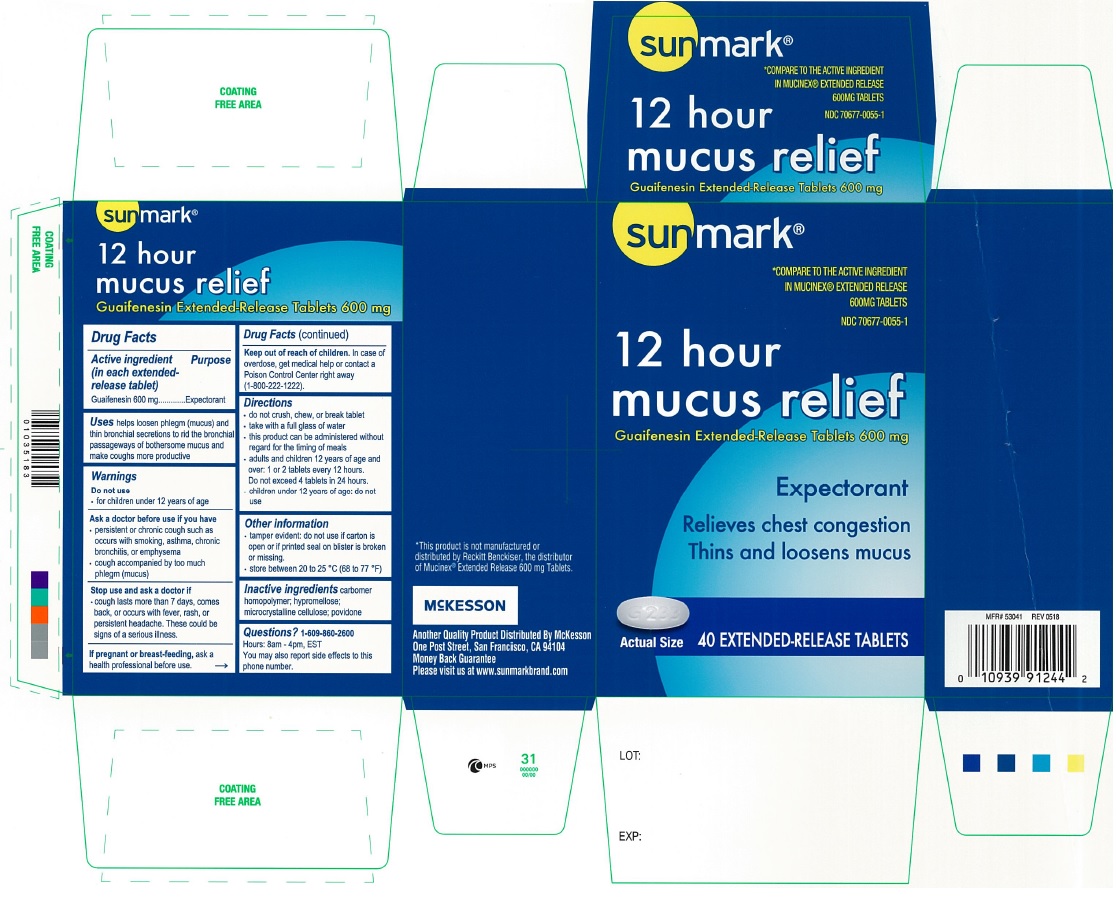
PRINCIPAL DISPLAY PANEL
sunmark
COMPARE TO THE ACTIVE INGREDIENT IN MUCINEX® EXTENDED RELEASE 600 MG TABLETSNDC: 70677-0055-1
12 hour
mucus reliefGuaifenesin Extended-Release Tablets 600 mg
Expectorant
Relieves chest congestion
Thins and Loosens Mucus
40 Extended-Release Tablets
-
INGREDIENTS AND APPEARANCE
SUNMARK MUCUS RELIEF
guaifenesin tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 70677-0055 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 600 mg Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) Product Characteristics Color WHITE Score no score Shape CAPSULE Size 22mm Flavor Imprint Code G233 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70677-0055-1 4 in 1 CARTON 09/26/2019 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA209215 07/22/2018 Labeler - Strategic Sourcing Services LLC (116956644)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.