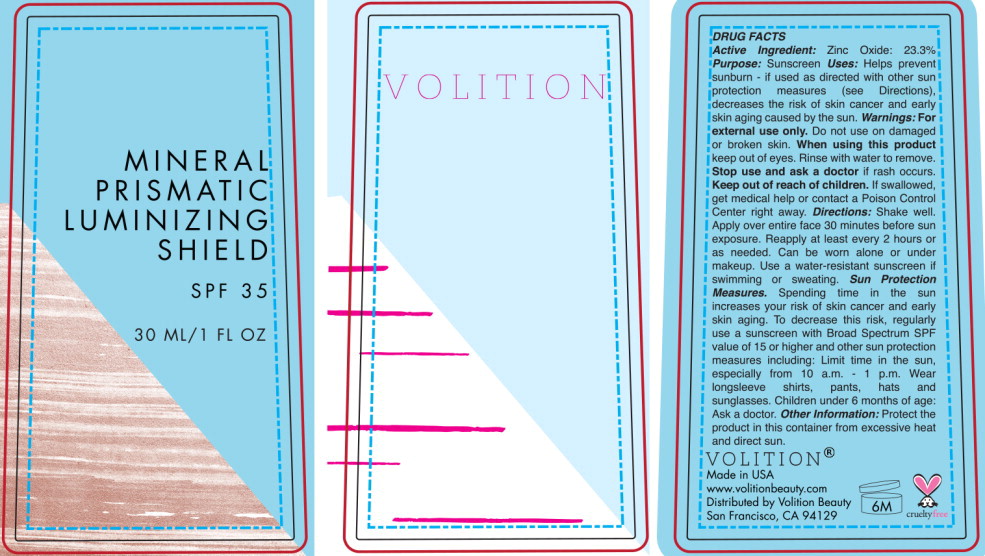
Mineral Prismatic Luminizing Shield SPF 35 by Volition Beauty Drug Facts
Mineral Prismatic Luminizing Shield SPF 35 by
Drug Labeling and Warnings
Mineral Prismatic Luminizing Shield SPF 35 by is a Otc medication manufactured, distributed, or labeled by Volition Beauty. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
MINERAL PRISMATIC LUMINIZING SHIELD SPF 35- zinc oxide liquid
Volition Beauty
----------
Drug Facts
Uses
Helps prevent sunburn if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
Directions
- Shake well. Apply over entire face 30 minutes before sun exposure.
- Reapply at least every 2 hours or as needed. Can be worn alone or under makeup.
- Use a water-resistant sunscreen if swimming or sweating.
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. -1 p.m.
- wear long-sleeve shirts, pants, hats and sunglasses
- children under 6 months of age: Ask a doctor.
Inactive Ingredients
Water/Aqua/Eau, Ethylhexyl Olivate, C13-15 Alkane, Butyloctyl Salicylate, Polyglyceryl-3 Polyricinoleate, Sorbitan Isostearate, Argania Spinosa Kernel Oil, Tocopheryl Acetate, Triethoxycaprylylsilane, Synthetic Fluorphlogopite, Glycerin, Sodium Chloride, Squalane, Butylene Glycol, Actinidia Chinensis (Kiwi) Fruit Water, Sodium Benzoate, Bisabolol, Potassium Sorbate, Alcohol, Sophora Flavescens Root Extract, Hydrolyzed Sodium Hyaluronate, Glycyrrhiza Glabra (Licorice) Root Extract, Ascorbic Acid, Sodium Ferrocyanide, Hydrogenated Lecithin, Iron Oxides (Cl 77499), Iron Oxides (Cl 77492), Iron Oxides (Cl 77491), Titanium Dioxide (Cl 77891).
| MINERAL PRISMATIC LUMINIZING SHIELD SPF 35
zinc oxide liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Volition Beauty (023668513) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.