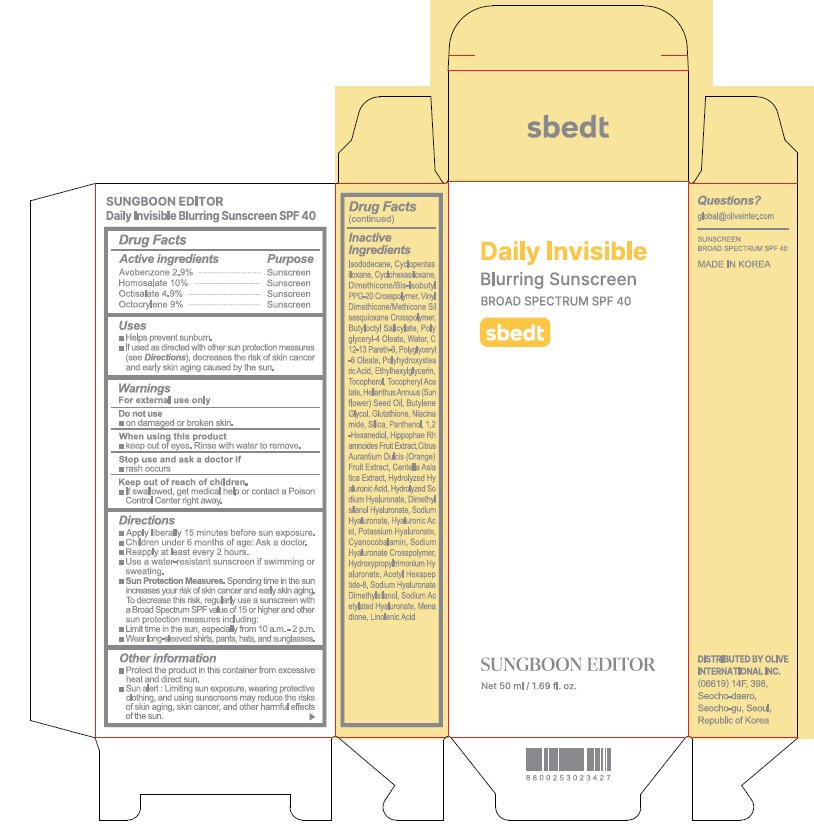
SUNGBOON EDITOR DAILY INVISIBLE BLURRING SUNSCREEN- avobenzone, homosalate, octisalate, octocrylene cream
Sungboon Editor Daily Invisible Blurring Sunscreen by
Drug Labeling and Warnings
Sungboon Editor Daily Invisible Blurring Sunscreen by is a Otc medication manufactured, distributed, or labeled by Olive International Inc., Rebom Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
DOSAGE & ADMINISTRATION
Apply liberally 15 minutes before sun exposure
Children under 6 months or age: Ask a doctor.
Reapply at least every two hours
Use a water-resistant sunscreen if swimming or sweating
Sun protection measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF or 15 of higher and other sun protection measures including:Limited time in the sun, especially from 10 am to 2 pm.
Wear long-sleeve shirts, pants, hats, and sunglasses
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
-
INACTIVE INGREDIENT
Isododecane, Cyclopentasiloxane, Cyclohexasiloxane, Dimethicone/Bis-Isobutyl PPG-20 Crosspolymer, Vinyl Dimethicone/Methicone Silsesquioxane Crosspolymer, Butyloctyl Salicylate, Polyglyceryl-4 Oleate, Water, C12-13 Pareth-9, Polyglyceryl-6 Oleate, Polyhydroxystearic Acid, Ethylhexylglycerin, Tocopherol, Tocopheryl Acetate, Helianthus Annuus (Sunflower) Seed Oil, Butylene Glycol, Glutathione, Niacinamide, Silica, Panthenol, 1,2-Hexanediol, Hippophae Rhamnoides Fruit Extract, Citrus Aurantium Dulcis (Orange) Fruit Extract, Centella Asiatica Extract, Hydrolyzed Hyaluronic Acid, Hydrolyzed Sodium Hyaluronate, Dimethylsilanol Hyaluronatem Sodium Hyaluronate, Hyaluronic Acid, Potassium Hyaluronate, Cyanocobalamin, Sodium Hyaluronate Crosspolymer, Hydroxypropyltrimonium Hyaluronate, Acetyl Hexapeptide-8, Sodium Hyaluronate Dimethylsilanol, Sodium Acetylated Hyaluronate, Menadione, Linolenic Acid
- OTHER SAFETY INFORMATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SUNGBOON EDITOR DAILY INVISIBLE BLURRING SUNSCREEN
avobenzone, homosalate, octisalate, octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 85037-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 29 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 100 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (ETHYLHEXYL SALICYLATE - UNII:4X49Y0596W) OCTISALATE 49 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 90 mg in 1 mL Inactive Ingredients Ingredient Name Strength 1,2-HEXANEDIOL (UNII: TR046Y3K1G) HIPPOPHAE RHAMNOIDES FRUIT (UNII: AVL0R9111T) ORANGE (UNII: 5EVU04N5QU) CENTELLA ASIATICA TRITERPENOIDS (UNII: 4YS74Q4G4J) DIMETHYLSILANOL HYALURONATE (UNII: Z853O1D4TE) HYALURONATE SODIUM (UNII: YSE9PPT4TH) HYALURONIC ACID (UNII: S270N0TRQY) CYANOCOBALAMIN (UNII: P6YC3EG204) ACETYL HEXAPEPTIDE-8 AMIDE (UNII: L4EL31FWIL) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) MENADIONE (UNII: 723JX6CXY5) LINOLENIC ACID (UNII: 0RBV727H71) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ISODODECANE (UNII: A8289P68Y2) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) DIMETHICONE/BIS-ISOBUTYL PPG-20 CROSSPOLYMER (UNII: O4I3UFO6ZF) VINYL DIMETHICONE/METHICONE SILSESQUIOXANE CROSSPOLYMER (UNII: 9NH1UDD2RR) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) POLYGLYCERYL-4 OLEATE (UNII: 15B05TY4GX) WATER (UNII: 059QF0KO0R) C12-13 PARETH-9 (UNII: 9BXD858P37) POLYGLYCERYL-6 OLEATE (UNII: G4AYJ54K59) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) TOCOPHEROL (UNII: R0ZB2556P8) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SUNFLOWER OIL (UNII: 3W1JG795YI) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) GLUTATHIONE (UNII: GAN16C9B8O) NIACINAMIDE (UNII: 25X51I8RD4) PANTHENOL (UNII: WV9CM0O67Z) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 85037-002-02 1 in 1 CARTON 03/25/2025 1 NDC: 85037-002-01 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/25/2025 Labeler - Olive International Inc. (695818354) Registrant - Olive International Inc. (695818354) Establishment Name Address ID/FEI Business Operations Rebom Co., Ltd. 695951708 manufacture(85037-002)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.