CANDIN- candida albicans skin test antigen injection, solution
Candin by
Drug Labeling and Warnings
Candin by is a Other medication manufactured, distributed, or labeled by Nielsen BioSciences, Inc., Allermed Laboratories, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CANDIN® safely and effectively. See full prescribing information for CANDIN.
Candida albicans Skin Test Antigen For Cellular Hypersensitivity
CANDIN intradermal injection
Initial U.S. Approval 1995
Nielsen Biosciences, Inc.
Manufactured by Allermed Laboratories, Inc.WARNING
See full prescribing information for complete boxed warning
- The expected response to CANDIN is a local area of inflammation at the site of the skin test. The size of reaction depends upon the sensitivity of the person receiving the test, but is usually dime to quarter size reaching maximum diameter between 24 and 48 hours. Larger accelerated reactions can occur which may require treatment with local cold compresses and anti-inflammatory medication. ( 2.3, 6.1)
- Systemic reactions can occur with skin test antigens and in certain individuals these reactions may be life-threatening or cause death. Emergency measures as well as personnel trained in their use should be immediately available in the event of a life-threatening reaction. Patients should be observed for at least 20 minutes following the administration of a skin test. ( 6.2)
- CANDIN should never be given intravenously. See also Warnings and Precautions. ( 5.1)
- Serious adverse reactions to CANDIN should be reported to Nielsen Biosciences, Inc. at (855) 855-1212 or MEDWATCH, Food and Drug Administration, 5600 Fishers Lane, Rockville, MD 20852-9782. Telephone: (800) 822-7967 or www.vaers.hhs.gov. ( 6.3)
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- CANDIN skin test dose is 0.1 mL administered intradermally. ( 2.1)
DOSAGE FORMS AND STRENGTHS
- CANDIN is a solution for intradermal injection supplied in a 1 mL multi-dose vial. ( 3.1)
- The skin-test strength of CANDIN has been determined from dose-response studies in healthy adults. The product is intended to elicit an induration response ≥ 5 mm in immunologically competent persons with cellular hypersensitivity to the antigen. ( 1, 14)
CONTRAINDICATIONS
- CANDIN should not be used after a previous unacceptable adverse reaction to this antigen or to a similar product, i.e., extreme hypersensitivity/allergy.
WARNINGS AND PRECAUTIONS
- The antigen must be injected intradermally. Do not inject intravenously. ( 5.1)
- As has been observed with other, unstandardized, antigens used for DTH skin testing, it is possible that some patients may have exquisite immediate hypersensitivity to CANDIN. ( 5.2)
- Physicians using this product must have facilities, equipment and medication necessary to treat potential side effects. Epinephrine and oxygen must be immediately available in the event of a serious systemic response. ( 5.3)
ADVERSE REACTIONS
- Immediate hypersensitivity local reactions can include itching, swelling, pain and blistering at the test site occurring 15-20 minutes after administration. Necrosis is possible. ( 6.1)
- Systemic reactions to CANDIN have not been observed, however all foreign antigens have the remote possibility of causing Type 1 anaphylaxis and even death when injected intradermally. ( 6.2)
- To report SUSPECTED ADVERSE REACTIONS, contact Nielsen Biosciences, Inc. at (855) 855-1212 or Food and Drug Administration (FDA) at 1-800-332-1088 or www.vaers.hhs.gov. ( 6.3)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING
1 INDICATIONS AND USAGE
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
6 ADVERSE REACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
13 NONCLINICAL TOXICOLOGY
14 CLINICAL STUDIES
14.1 Response to CANDIN IN Healthy Adults
14.2 Cellular hypersensitivity response to CANDIN in adults with AIDS, adults with HIV infection (no-AIDS-indicator conditions) and adult control subjects (Table 2)
14.3 Cellular hypersensitivity response to CANDIN in adults with cancer (Table 3)
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING
- The expected response to CANDIN is a local area of inflammation at the site of the skin test. The size of reaction depends upon the sensitivity of the person receiving the test, but is usually dime to quarter size reaching maximum diameter between 24 and 48 hours. Larger accelerated reactions can occur which may require treatment with local cold compresses and anti-inflammatory medication. ( 2.3, 6.1)
- Systemic reactions can occur with skin test antigens and in certain individuals these reactions may be life-threatening or cause death. Emergency measures as well as personnel trained in their use should be immediately available in the event of a life-threatening reaction. Patients should be observed for at least 20 minutes following the administration of a skin test. ( 6.2)
- CANDIN should never be given intravenously. See also Warnings and Precautions. ( 5.1)
- Serious adverse reactions to CANDIN should be reported to Nielsen Biosciences, Inc. at (855) 855-1212 or MEDWATCH, Food and Drug Administration, 5600 Fishers Lane, Rockville, MD 20852-9782. Telephone: (800) 332-1088 or www.vaers.hhs.gov. ( 6.3)
-
1 INDICATIONS AND USAGE
CANDIN is indicated for use as a recall antigen for detecting cell-mediated hypersensitivity by intracutaneous (intradermal) testing. The product may be useful in evaluating the cellular immune response in patients suspected of having reduced cellular hypersensitivity. Because some persons with normal cellular immunity are not hypersensitive to Candida, a response rate less than 100% to the antigen is to be expected in normal individuals. Therefore, the concurrent use of other licensed cell-mediated hypersensitivity skin test antigens is recommended. The product should not be used to diagnose or treat Type 1 allergy to Candida albicans.
The potency of CANDIN is measured by DTH skin tests in humans. The procedure involves concurrent (side-by-side) testing of production lots with an Internal Reference (IR), using sensitive adults who have been previously screened and qualified to serve as test subjects. The induration response at 48 hours elicited by 0.1 mL of a production lot is measured and compared to the response elicited by 0.1 mL of the IR. The test is satisfactory if the potency of the production lot does not differ more than ± 20% from the potency of the IR, when analyzed by the paired t-test (two-tailed) at a p value of 0.05.
The potency of the IR is monitored by DTH skin testing. Persons included in the potency assay are qualified as test subjects by receiving four skin tests with the IR from which a mean induration response (mm) is calculated. Current skin tests with the IR must show that the potency of the IR has not changed more than ± 20% from the mean qualifying response in the same test subjects, when analyzed by the paired t-test (two-tailed) at a p value of 0.05. The required induration response at 48 hours to the IR is 15 mm ± 20%.
- 2 DOSAGE AND ADMINISTRATION
- SPL UNCLASSIFIED SECTION
-
SPL UNCLASSIFIED SECTION
2.2 CANDIN should be administered intradermally on the volar surface of the forearm or on the outer aspect of the upper arm. The test dose is 0.1 mL. The skin should be cleansed with 70% alcohol before applying the skin test. The intradermal injection must be given as superficially as possible causing a distinct, sharply defined bleb. An unreliable reaction may result if the product is injected subcutaneously. A positive DTH reaction to CANDIN consists of induration ≥ 5 mm.
-
SPL UNCLASSIFIED SECTION
2.3 Interpretation of Skin Test: The time required for the induration response to reach maximum intensity varies with the individual. The reaction usually begins within 24 hours and peaks between 24 and 48 hours. The skin test should be read at 48 hours by visually inspecting the test site and palpating the indurated area. Measurements should be made across two diameters as shown in the figure below. The mean of the longest and midpoint orthogonal diameters of the indurated area should be reported as the DTH response. For example, a reaction that is 10 mm (longest diameter) by 8 mm (midpoint orthogonal diameter) has a sum of 18 mm and a mean of 9 mm. The DTH response is therefore 9 mm.
Area of induration (shaded area)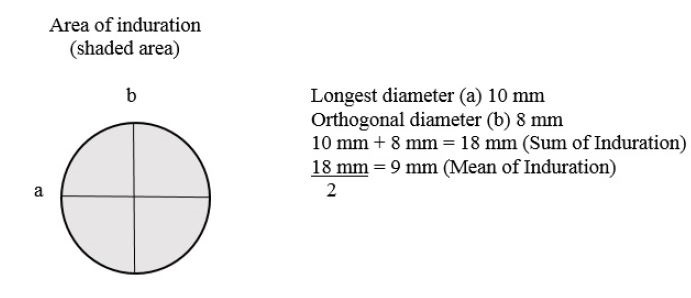
- 3 DOSAGE FORMS AND STRENGTHS
- SPL UNCLASSIFIED SECTION
-
SPL UNCLASSIFIED SECTION
3.2 The skin-test strength of CANDIN has been determined from dose-response studies in healthy adults [see CLINICAL STUDIES ( 14)]. The product is intended to elicit an induration response ≥ 5 mm in immunologically competent persons with cellular hypersensitivity to the antigen [see DOSAGE AND ADMINISTRATION ( 2.2)].
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 The antigen must be injected intradermally as superficially as possible, causing a distinct, sharply defined bleb at the skin test site. An unreliable reaction may result if the product is injected subcutaneously. It must not be given intravenously; care should be taken to avoid injection into a blood vessel. A separate sterile syringe and needle should be used for each patient to prevent transmission of infectious agents. Needles should be disposed of properly and should not be recapped.
5.2 As has been observed with other, unstandardized, antigens used for DTH skin testing (10), it is possible that some patients may have exquisite immediate hypersensitivity to CANDIN. In persons with bleeding tendency, bruising and non-specific induration may occur due to the trauma of the skin test. As with all skin test antigens, local and systemic allergic reactions can occur following administration.
-
6 ADVERSE REACTIONS
6.1 Local reactions to CANDIN have included swelling, pruritus and vesiculation. Reactions involving necrosis and ulceration have not been observed, but such reactions are theoretically possible and might occur in persons with exquisite cellular hypersensitivity to the antigen. Local reactions may be treated with a cold compress and topical steroids. Severe local reactions may require additional measures as appropriate.
In a published study of 479 HIV positive adults tested with CANDIN, adverse local reactions were observed in six subjects as follows: pruritus (three), swelling at the test site (one), vesiculation (one) and vesiculation with weeping edema (one). Pruritus and swelling cleared within 48 hours; vesiculation with edema required approximately 1 week to resolve 6
In two studies involving 171 persons discussed under CLINICAL STUDIES in Tables 1, 2, 3, and text, one adverse reaction was observed. This reaction consisted of induration 22 x 55 mm at 48 hours which resolved within 1 week 8.
Testing of CANDIN for consistency of potency is performed in healthy human subjects who are known to be skin-test positive to the antigen. In 58 subjects tested to-date, there have been no cases of Type 1 allergy manifested as either generalized or adverse local reactions. One subject had induration with a central vesicle which subsided within a few days 8.
Severe local reactions, including rash, vesiculation, bullae, dermal exfoliation and cellulitis, have been reported to MedWatch for unstandardized allergenic extracts of Candida albicans used for anergy testing
6.2 Systemic reactions to CANDIN have not been observed. However, all foreign antigens have the remote possibility of causing Type 1 anaphylaxis (7) and even death when injected intradermally. Systemic reactions usually occur within 30 minutes after the injection of antigen and may include the following symptoms: sneezing, coughing, itching, shortness of breath, abdominal cramps, vomiting, diarrhea, tachycardia, hypotension and respiratory failure in severe cases. Systemic allergic reactions including anaphylaxis must be immediately treated with Epinephrine HCL 1:1,000. Additional measures may be required, depending upon the severity of the reaction.
Immediate Hypersensitivity reactions to CANDIN occur in some individuals. These reactions are characterized by the presence of an edematous hive surrounded by a zone of erythema. They occur approximately 15 - 20 minutes after the intradermal injection of the antigen. The size of the immediate reaction varies depending upon the sensitivity of the individual. Immediate hypersensitivity reactions were observed in the control and HIV-infected (AIDS and HIV positive) subjects reported in Table 2 as follows: HIV-infected subjects (20% with erythema of 10 - 21 mm in diameter; 13% with erythema of 5 - 9 mm). Control subjects (22% with erythema of 10 - 15 mm; 5% with erythema of 8.5 mm). Cancer subjects (Group 1, Table 3), 17% with erythema of 10 - 24 mm and 11% with erythema of 6 - 9 mm.
- 7 DRUG INTERACTIONS
-
SPL UNCLASSIFIED SECTION
7.1 Pharmacologic doses of corticosteroids may variably suppress the inflammatory response to DTH skin test antigen after two weeks of therapy. may variably suppress the DTH skin test response after two weeks of therapy. The mechanism of suppression is believed to involve a decrease in monocytes and lymphocytes, particularly T-cells. The skin test response usually returns to the pretreatment level within several weeks after steroid therapy is discontinued 1.
- SPL UNCLASSIFIED SECTION
-
8 USE IN SPECIFIC POPULATIONS
8.3 Nursing Mothers
It is not known whether CANDIN is excreted in human milk. Because drugs may be excreted in human milk, caution should be exercised when this product is administered to a nursing woman.
-
10 OVERDOSAGE
The recommended dose for CANDIN is 0.1 mL administered intradermally. If a larger dose is administered, or if the dose is accidentally injected intravenously, the potential for a systemic reaction such as anaphylaxis is increased. In this case, Epinephrine HCl at 1:1000 should be made available immediately as well as oxygen and emergency equipment.
Because individuals differ in their sensitivity to CANDIN®, the response to the recommended dose of 0.1 mL can vary in size and intensity. In highly sensitive persons, the result of overdose, or a mistake in the administration of CANDIN may result in a more pronounced local or systemic outcome.
-
11 DESCRIPTION
Candida albicans Skin Test Antigen for Cellular Hypersensitivity (CANDIN) is a clear, colorless, sterile solution with a pH of 8.0 - 8.5. The antigen should be administered intradermally according to the directions included under DOSAGE AND ADMINISTRATION ( 2.2) of this package insert.
CANDIN is made from the culture filtrate and cells of two strains of Candida albicans. The fungi are propagated in a chemically defined medium consisting of inorganic salts, biotin and sucrose. Lyophilized source material is extracted with a solution of 0.25% NaCl, 0.125% NaHCO3 and 50% v/v glycerol. The concentrated extract is diluted with a solution of 0.5% NaCl, 0.25% NaHCO3, 0.03% Albumin (Human) USP, 8 ppm polysorbate 80 and 0.4% phenol.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Cellular hypersensitivity or delayed-type hypersensitivity (DTH) can be assessed by intracutaneous testing with bacterial, viral and fungal antigens to which most healthy persons are sensitized. A positive skin test denotes prior antigenic exposure, T-cell competency and an intact inflammatory response 1, 2. The reaction usually peaks between 24 and 48 hours after antigen is introduced into the skin and is manifest as induration at the test site.
12.2 Pharmacodynamics
The inflammatory response associated with the DTH reaction is characterized by an infiltration of lymphocytes and macrophages at the site of antigen deposition. Specific cell types that appear to play a major role in the DTH response include CD4+ and CD8+ T lymphocytes which leave the recirculating lymphocyte pool in response to exogenous antigen 3. Both CD4+ and CD8+ lymphocytes have been recovered from DTH reactions elicited by Candida antigen 4.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
14.1 Response to CANDIN IN Healthy Adults
In one group of 18 subjects, 14 (78%) of the individuals reacted to CANDIN with an induration response of ≥ 5 mm at 48 hours. In a second study of 35 subjects, 21 (60%) had induration reactions ≥ 5 mm at 48 hours. In this study, 65% of males tested positive compared to 53% of females; the mean induration in responding males was 12.8 mm and in responding females was 13.0 mm.
14.2 Cellular hypersensitivity response to CANDIN in adults with AIDS, adults with HIV infection (no-AIDS-indicator conditions) and adult control subjects (Table 2)
Response to CANDIN in Adults with HIV Infection: In one study (Table 2), the skin test responses of adults with HIV infection were compared to those of healthy control subjects (age range AIDS 22 - 65, HIV positive 20 - 45, Controls 25 - 69). When HIV-infected subjects were classified by the CDC's 1993 revised classification system for HIV infection(5), a significant difference was found between AIDS patients and normal controls in both mean induration (p = 0.01) and proportion with ≥ 5 mm response (p > 0.01). The responses in HIV-infected patients (without AIDS-indicating conditions or AIDS-indicating CD4 T-cell counts) were less than in normal subjects, but the differences were not statistically significant.
In a second study involving 20 male patients (age range 26 - 57) diagnosed with AIDS based on clinical criteria only, one subject responded to CANDIN. In the same study 65% of the male control subjects had DTH reactions ≥ 5 mm to CANDIN (Table 1, Study 2). The mean induration response at 48 hours for control subjects was 8.33 mm compared to 1.78 mm for the AIDS subject. AIDS vs. control p-values were < 0.01 mean induration and < 0.01 induration ≥ 5 mm.
Because HIV infection can modify the DTH response to tuberculin, it is advisable to skin test HIV-infected patients at high risk of tuberculosis with antigens in addition to tuberculin (6). In a published study of DTH anergy, 479 subjects (334 males and 145 females) infected with HIV and being screened for tuberculosis were skin tested with several additional antigens, including CANDIN supplied under IND to the investigators. Only 12% reacted to tuberculin (≥ 5 mm), 57% reacted to CANDIN (≥ 3 mm) and 60% reacted to either tuberculin or CANDIN or both. In this study, a 3 mm induration response to CANDIN was considered positive. The authors concluded that in HIV-infected subjects, testing with other DTH antigens increases the accuracy of interpretation of negative tuberculin reactions.
Table 2. Cellular hypersensitivity response to CANDIN in adults with AIDS, adults with HIV infection (no-AIDS-indicator conditions) and adult control subjects. CD 4 T-cell Count Group Classifi-
cation* *N Zidov-
udine
UseRange Mean Mean
Indur-
ation
(mm)N≥5
(mm)% - * (reference 12)
- † p=0.01 compared to Control.
- ‡ p<0.01 compared to Control.
AIDS A3,B3,C 32 14 4-483 145 3.35 † 9 28 ‡ HIV
POS.A1,A2,
B1,B228 13 201-1065 455 5.67 15 54 Control ---- 18 0 554-1876 869 8.03 14 78 -
14.3 Cellular hypersensitivity response to CANDIN in adults with cancer (Table 3)
IIn one study of 18 patients with lung cancer, CANDIN elicited a positive induration response in five patients (28%). In a second series of 20 patients with metastatic cancer, no reactions ≥5 mm were observed (Table 3).
Table 3. Cellular hypersensitivity response to CANDIN in adults with cancer. N Age
rangeNumber reactions
≥ 5 mm at 48 hoursResponse Study 1 18 52-75 5 28% Study 2 20 47-81 0 0% -
15 REFERENCES
- Middleton, E. Jr., Reed, C.E., Ellis, F.E., Adkinson, N.F., Jr., Yunginger, J.W., Busse, W.W., Allergy Principles and Practice, 4th Ed., Vol II, pp 963-982, Mosby, St. Louis, 1993.
- Bernstein, I.L., ed., Proceedings of the task force of guidelines for standardizing old and new technologies used for the diagnosis and treatment of allergy, J. Allergy Clin. Immunol., 82: 487-526, 1988.
- Paul, W.E., Fundamental Immunology, 3rd Ed., pp 75-76, Raven Press, New York, 1993.
- MacPhee, M.J., Gordon, J., Christou, N.V., Sanchez-Cantu, L., Rode, H.H., Cells recovered from human DTH reactions: phenotypic and functional analysis. Cellular Immunology, 151: 80-96, 1993.
- CDC, 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morbidity and Mortality Weekly Report. 41: No. RR17, December 18, 1992.
- Huebner, R.E., Schein, M.F., Hall, C.A., Barnes, S.A., Delayed-type hypersensitivity anergy in human immunodeficiency virus-infected persons screened for infection with Mycobacterium tuberculosis. Clin. Infect. Dis., 19: 26-32, 1994
- Klotz, S.D., Sweeney, M.J., Dienst, S., Klotz, L.R., Moeller, R.K., Rosenberg, S., Systemic anaphylaxis immediately following delayed hypersensitivity skin tests. Ann. Allergy, 49: 142-144, 1982
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Local reactions to CANDIN can include redness, swelling, pruritus, excoriation and discoloration of the skin. These reactions usually subside within hours or days after administration of the skin test. In some patients, skin discoloration may persist for several weeks. Progression of the DTH reaction to vesiculation, necrosis and ulceration are possible. Patients should be informed that all foreign antigens have the remote possibility of causing Type I anaphylactic reactions that may require the administration of epinephrine and other drugs or emergency procedures and may be life threatening in some cases. Patients should report any serious adverse reactions to their health care provider.
∗Sections or subsections omitted from the full prescribing information are not listed.
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - VIAL
Candida albicans Skin Test Antigen For Cellular Hypersensitivity
CANDIN
NDC: 59584-138-01 1D barcode: 59584-138-01
Volume: 1 mL (10 Tests)
Dose: 0.1 mL Intradermally
See Package Insert
Store: 2-8 °C Rx Only
Preservative: 0.4% PHENOL
Mfd. for Nielsen BioSciences, Inc.
San Diego CA 921212D barcode
Lot:
Exp:
-
PRINCIPAL DISPLAY PANEL
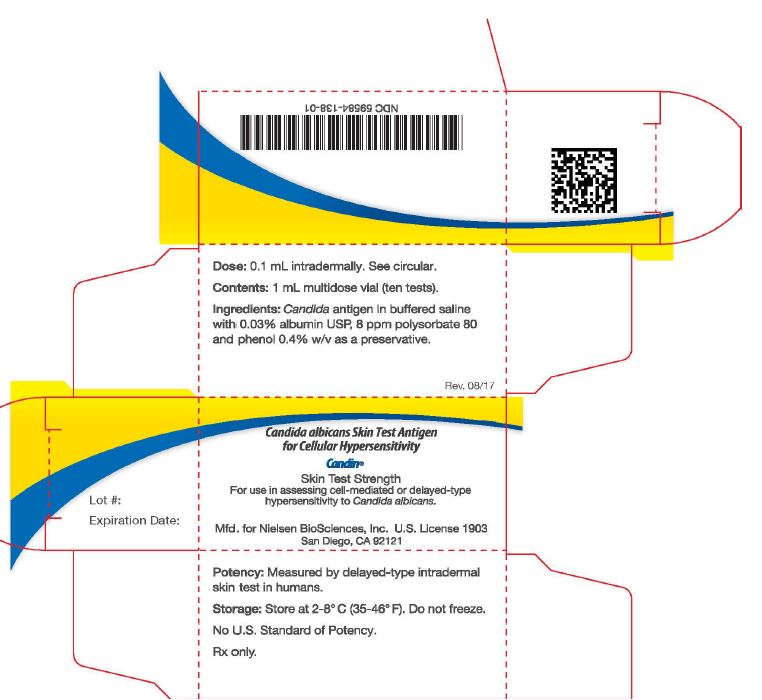
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - CARTON
DOSE: 0.1 mL intradermally. See circular. (panel 1)
CONTENTS: 1 mL multidose vial (ten tests).
INGREDIENTS: Candida antigen in buffered saline with 0.03% albumin USP, 8ppm polysorbate 80 and phenol 0.4% as a preservative.
Rev 9/15
Lot: CAxxx (on end flap)
Expiration Date: (on end flap)Candida albicans Skin Test Antigen to Assess Cellular Hypersensitivity (panel 2)
CANDINSkin Test Strength
For use in assessing cell-mediated or delayed-type
hypersensitivity to Candida albicans.Mfd. for Nielsen BioSciences, Inc., U.S. License 1903
San Diego, CA 92121
Potency: Measured by delayed-type intradermal
skin tests in humans. (panel 3)Storage: Store at 2-8° C (35-46° F). Do not freeze.
No U.S. Standard of Potency.
Rx only.
1D barcode: 59584-138-01 (4th panel)
2D GS1 serialized barcode (on end flap)
-
INGREDIENTS AND APPEARANCE
CANDIN
candida albicans skin test antigen injection, solutionProduct Information Product Type STANDARDIZED ALLERGENIC Item Code (Source) NDC: 59584-138 Route of Administration INTRADERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CANDIDA ALBICANS (UNII: 4D7G21HDBC) (CANDIDA ALBICANS - UNII:4D7G21HDBC) CANDIDA ALBICANS 1 U in 0.1 mL Inactive Ingredients Ingredient Name Strength ALBUMIN HUMAN (UNII: ZIF514RVZR) 0.03 mg in 0.1 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 0.5 mg in 0.1 mL SODIUM BICARBONATE (UNII: 8MDF5V39QO) 0.25 mg in 0.1 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) 0.8 ug in 0.1 mL PHENOL (UNII: 339NCG44TV) 0.4 mg in 0.1 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59584-138-01 1 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA103257 12/01/1995 Labeler - Nielsen BioSciences, Inc. (078835288) Registrant - Nielsen BioSciences, Inc. (078835288) Establishment Name Address ID/FEI Business Operations Allermed Laboratories, Inc. 073364531 manufacture Establishment Name Address ID/FEI Business Operations Nielsen BioSciences, Inc. 078835288 manufacture
Trademark Results [Candin]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CANDIN 74351614 1970269 Live/Registered |
NIELSEN BIOSCIENCES, INC. 1993-01-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.