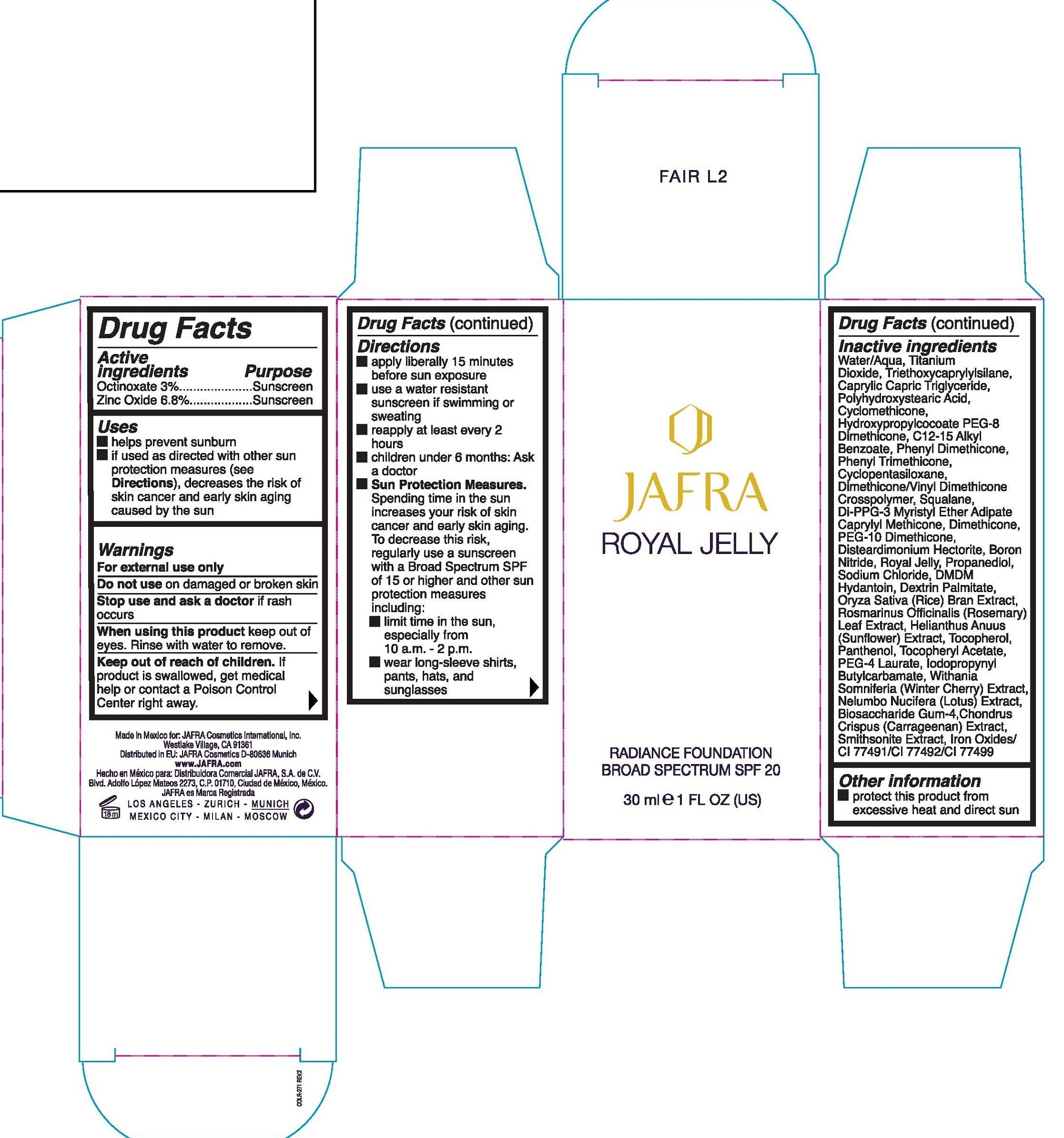
JAFRA ROYAL JELLY RADIANCE BROAD SPECTRUM SPF 20 BARE L6- octinoxate, zinc oxide emulsion
Absara Cosmetics S.A.P.I DE C.V.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredients
Octinoxate 3%
Zinc Oxide 6.8%
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see
Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Warnings
For external use only
Do not use
on damaged or broken skin
Stop use and ask a doctor
if rash occurs
When using this product
keep out of eyes. Rinse with water to remove.
Keep out of reach of children.
If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions
- apply liberally 15 minutes before use exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- children under 6 months: Ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. -2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
Inactive ingredients
Water/Aqua, Titanium Dioxide, Triethoxycaprylylsilane, Caprylic, Capric Triglyceride, Polyhydroxystearic Acid, Cyclomethicone, Hydroxypropylcocoate PEG-8 Dimethicone, C12-15 Alkyl Benzoate, Phenyl Dimethicone, Phenyl Trimethicone, Cyclopentasiloxane, Dimethicone/Vinyl Dimethicone Crosspolymer, Squalane, Di-PPG-3 Myristyl Ether Adipate Caprylyl Methicone, Dimethicone, PEG-10 Dimethicone, Dissteardimonium Hectorite, Boron Nitride, Royal Jelly, Propanediol, Sodium Chloride, DMDM Hyantoin, Dextrin Palmitate, Oryza Sativa (Rice) Bran Extract, Rosmarinus Officinalis (Rosemary) Leaf Extract, Helianthus Anuus (Sunflower) Extract, Tocopherol, Panthenol, Tocopheryl Acetate, PEG-4 Laurate, Iodopropynyl Butylcarbamate, Withania Somniferia (Winter Cherry) Extract, Biosaccharide Gum-4, Chlndrus Crispus (Carrageenan) Extract, Smithsonite Extract, Iron Oxides/ CI 77491/CI 77492/CI 77499
Other information
protect this product from excessive heat and direct sun
Package Labeling: