Odacite Sun Guardian Oceanic Glacial Water Day Creme SPF30
Odacite Sun Guardian Oceanic Glacial Water Day Creme SPF30 by
Drug Labeling and Warnings
Odacite Sun Guardian Oceanic Glacial Water Day Creme SPF30 by is a Otc medication manufactured, distributed, or labeled by Odacite Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
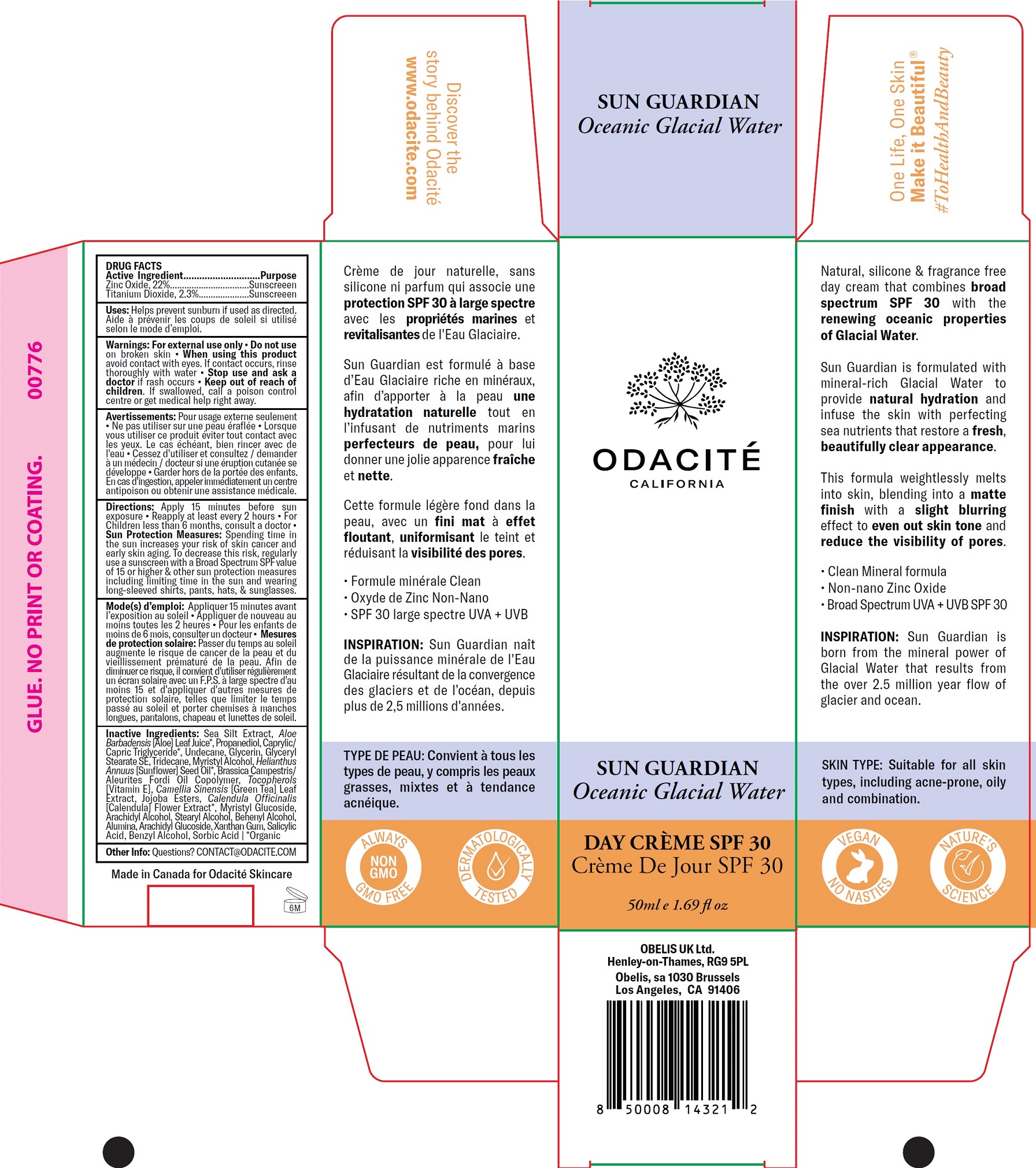
ODACITE SUN GUARDIAN OCEANIC GLACIAL WATER DAY CREME SPF30- zinc oxide, titanium dioxide cream
Odacite Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Odacite Sun Guardian Oceanic Glacial Water Day Creme SPF30
Directions:
Apply 15 minutes before sun exposure
- Reapply at least every 2 hours
- For Children less than 6 months, consult a doctor
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher & other sun protection measures including limiting time in the sun and wearing long-sleeved shirts, pants, hats, & sunglasses.
Inactive Ingredients:
Sea Silt Extract, Aloe Barbadensis [Aloe] Leaf Juice*, Propanediol, Caprylic/ Capric Triglyceride*, Undecane, Glycerin, Glyceryl Stearate SE, Tridecane, Myristyl Alcohol, Helianthus Annuus [Sunflower] Seed Oil*, Brassica Campestris/ Aleurites Fordi Oil Copolymer, Tocopherols [Vitamin E], Camellia Sinensis [Green Tea] Leaf Extract, Jojoba Esters, Calendula Officinalis [Calendula] Flower Extract*, Myristyl Glucoside, Arachidyl Alcohol, Stearyl Alcohol, Behenyl Alcohol, Alumina, Arachidyl Glucoside, Xanthan Gum, Salicylic Acid, Benzyl Alcohol, Sorbic Acid | *Organic
| ODACITE SUN GUARDIAN OCEANIC GLACIAL WATER DAY CREME SPF30
zinc oxide, titanium dioxide cream |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Odacite Inc (079682742) |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.