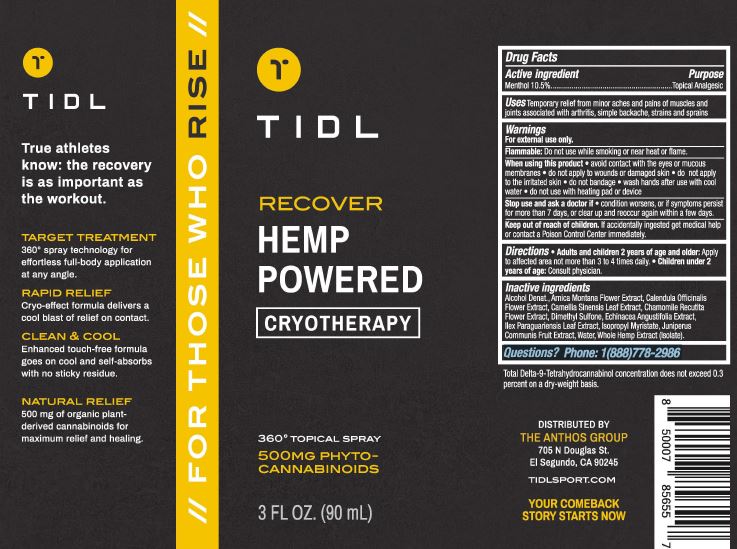
Recover Hemp Powered by The Anthos Group / Inspec Solutions LLC
Recover Hemp Powered by
Drug Labeling and Warnings
Recover Hemp Powered by is a Otc medication manufactured, distributed, or labeled by The Anthos Group, Inspec Solutions LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
RECOVER HEMP POWERED TIDL- menthol 10.5% spray
The Anthos Group
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
| RECOVER HEMP POWERED
TIDL
menthol 10.5% spray |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - The Anthos Group (117511051) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Inspec Solutions LLC | 081030372 | manufacture(79740-010) | |
Revised: 8/2020
Document Id: ac105d11-3df0-7f46-e053-2995a90ac2e5
Set id: 42fce5b6-be87-45c7-80eb-5ea6c9112b86
Version: 2
Effective Time: 20200804
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.