EQUISUL-SDT- sulfadiazine and trimethoprim suspension
Equisul-SDT by
Drug Labeling and Warnings
Equisul-SDT by is a Animal medication manufactured, distributed, or labeled by Aurora Pharmaceutical, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- CAUTION
-
DESCRIPTION
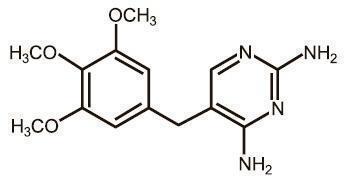
EQUISUL-SDT is a broad-spectrum antimicrobial from the potentiated sulfonamide class of chemotherapeutic agents. These two drugs block different sequential steps in the biosynthesis of nucleic acids. Sulfadiazine inhibits bacterial synthesis of dihydrofolic acid by competing with para-aminobenzoic acid. Trimethoprim blocks the production of tetrahydrofolic acid from dihydrofolic acid by reversibly inhibiting dihydrofolate reductase. The effect of the dual action is to reduce the minimum inhibitory concentration of each agent (synergism) and to convert a bacteriostatic action to a bactericidal action. Sulfadiazine is the non-proprietary name for 4-amino-N-2-pyrimidinylbenzenesulfonamide. Trimethoprim is the non-proprietary name for 5-[(3,4,5¬trimethoxyphenyl) methyl]-2,4-pyrimidinediamine.
Figure 1. Structure of sulfadiazine
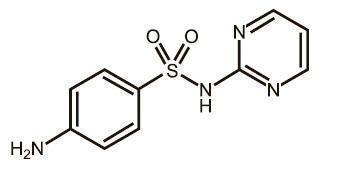
Figure 2. Structure of trimethoprim
Each mL of EQUISUL-SDT contains 400 mg combined active ingredients (333 mg sulfadiazine and 67 mg trimethoprim) in an aqueous suspension.
- INDICATION
- DOSAGE AND ADMINISTRATION
- CONTRAINDICATIONS
- WARNING
-
HUMAN WARNINGS
Not for use in humans. For use in animals only. Keep this and all drugs out of the reach of children. Consult a physician in the case of accidental human exposure.
Antimicrobial drugs, including sulfonamides, can cause mild to severe allergic reactions in some individuals. Avoid direct contact of the product with the skin, eyes, mouth, and clothing. Persons with a known sensitivity to sulfonamides or trimethoprim should avoid exposure to this product. If an allergic reaction occurs (e.g., skin rash, hives, difficulty breathing, facial swelling) seek medical attention.
-
PRECAUTIONS
Prescribing antibacterial drugs in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to treated animals and may increase the risk of development of drug-resistant animal pathogens.
The administration of antimicrobials, including sulfadiazine and trimethoprim, to horses under conditions of stress may be associated with acute diarrhea that can be fatal. If acute diarrhea or persistent changes in fecal consistency are observed, additional doses of EQUISUL-SDT should not be administered and appropriate therapy should be initiated.
The safe use of EQUISUL-SDT has not been evaluated in breeding, pregnant, or lactating horses. Potentiated sulfonamides should only be used in pregnant or lactating mares when the benefits to the mare justify the risks to the fetus. Use of potentiated sulfonamides during pregnancy has been associated with an increased risk of congenital abnormalities that may be related to folate deficiency. In humans, sulfonamides pass through the placenta, are excreted in milk, and may cause hyperbilirubinemia-induced neurotoxicity in nursing neonates.
Decreased hematopoetic activity and blood dyscrasias have been associated with the use of elevated doses and/or prolonged administration of potentiated sulfonamides. EQUISUL-SDT should be discontinued if prolonged clotting times, or decreased platelet, white blood cell or red blood cell counts are observed.
Sulfonamides should be used with caution in horses with impaired hepatic function. Although rare, sulfonamide use has been associated with fulminant hepatic necrosis in humans.
Neurologic abnormalities have been reported in several species following administration of potentiated sulfonamides. In horses, potentiated sulfonamides have been associated with gait alterations and behavior changes that resolved after discontinuation of the drug.
The safe use of EQUISUL-SDT has not been evaluated in horses less than 1 year of age.
-
ADVERSE REACTIONS
Adverse reactions reported during a field study of 270 horses of various breeds, ranging from 1 to 25 years of age, which had been treated with either EQUISUL-SDT (n = 182) or with a saline control (n = 88) are summarized in Table 1. At least one episode of loose stool of varying severity was observed in 69 of 182 (38%) of the EQUISUL-SDT-treated horses, and 29 of 88 (33%) saline control horses. Of those animals experiencing loose stool, 2 of 182 (1.1%) of the EQUISUL-SDT-treated horses and 0 of 88 (0%) placebo-treated horses were removed from the study due to diarrhea (defined as at least one episode of watery stool). Both cases of diarrhea in this study were self-limiting and resolved without treatment within 5–10 days after discontinuation of EQUISUL-SDT.
Table 1. Number of Horses with Adverse Reactions During the Field Study with EQUISUL-SDT Adverse Reactions Equisul-SDT
(n=182)Saline control
(n=88)Loose stool (including diarrhea) 69 (38%) 29 (33%) Colic 3 (1.6%) 2 (2.2%) Diarrhea 2 (1.1%) 0 (0%) To report suspected adverse events, for technical assistance or to obtain a copy of the MSDS, contact Aurora Pharmaceutical, Inc. at 888-215-1256 or www.aurorapharmaceutical.com. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at http://www.fda. gov/AnimalVeterinary/SafetyHealth.
-
CLINICAL PHARMACOLOGY
Following oral administration, EQUISUL-SDT is rapidly absorbed and widely distributed throughout body tissues. Sulfadiazine levels are usually highest in the kidney, while the tissue concentration in other tissues is only slightly lower than plasma concentrations. Concentrations of trimethoprim are usually higher in the lungs, kidney, and liver than in the blood. Sulfadiazine and trimethoprim are both eliminated primarily by renal excretion, both by glomerular filtration and tubular secretion. Urine concentrations of both sulfadiazine and trimethoprim are several-fold higher than blood concentrations.1 Sulfadiazine and trimethoprim are 20% and 35% bound to plasma protein, respectively. Administration of sulfadiazine and trimethoprim with food has no apparent effect on absorption of sulfadiazine but the absorption of trimethoprim is decreased.
Based on a study in fed horses, trimethoprim concentrations following repeat oral administration of 24 mg/kg EQUISUL-SDT to 6 horses reached peak concentration in 0.5 to 12.0 hours. The median plasma elimination half-life was 3 hours, with a range of 2.31 to 4.96 hours. Peak sulfadiazine concentrations were reached within 1.0 to 12.0 hours in the same study. The median plasma elimination half-life for sulfadiazine was approximately 7.80 hours, with a range of 6.78 to 10.39 hours. Only minor accumulation of both drugs was observed following repeat oral administration of EQUISUL-SDT and both drugs reached steady state by day 3. Sulfadiazine and trimethoprim key steady state parameters associated with administration in 6 fed horses over a period of 7 days are found in Table 2.
Table 2. Median (Range) of sulfadiazine and trimethoprim pharmacokinetics parameters following repeat dosing of 24 mg/kg bid EQUISUL-SDT for 7 days to six horses in fed condition Drug Sulfadiazine Trimethoprim Tmax (hr) 4.75
(1.0012.00)8.50
(0.5012.00)Cmax
(µg/mL)17.63
(10.1031.15)0.78
(0.601.14)AUC 012
(last dose)
(hr*µg/mL)159.35
(73.90282.54)5.47
(3.3110.91)T 1/2
(hr)7.80
(6.7810.39)3.00
(2.314.96)
- 1 Kahn CM, Line S, eds. The Merck Veterinary Manual. 10th Ed. Merck & Co. 2010.
MICROBIOLOGY
EQUISUL-SDT is the combination of the sulfonamide sulfadiazine and trimethoprim. These two drugs block sequential steps in nucleic acids biosynthesis. Sulfadiazine inhibits bacterial synthesis of dihydrofolic acid by competing with para-aminobenzoic acid. Trimethoprim blocks the production of tetrahydrofolic acid from dihydrofolic acid by reversibly inhibiting dihydrofolate reductase. The two drugs act synergistically, reducing the minimum inhibitory concentration of each, while enhancing the bacteriostatic action of each separately to a bactericidal action when combined.
EQUISUL-SDT administered as a combined sulfadiazine-trimethoprim dose of 24 mg/kg body weight twice daily for 7 days provided concentrations of sulfadiazine and trimethoprim with T>MIC90 (%T) values of 100% and 98% respectively. The minimum inhibitory concentration (MIC) values for EQUISUL-SDT against indicated pathogens isolated from lower respiratory tract infections in horses enrolled in a 2010–2011 effectiveness field study are presented in Table 3. All MICs were determined in accordance with the Clinical and Laboratory Standards Institute (CLSI) Approved Standard M31-A3 using a broth microdilution system and 3% lysed horse blood.
Table 3. Trimethoprim/sulfadiazine minimum inhibitory concentration (MIC) values* of isolates recovered from horses with lower respiratory infection caused by Streptococcus equi subsp. zooepidemicus treated with EQUISUL-SDT in the U.S. (2010–2011) Treatment Outcome Success Failure - * The correlation between in vitro susceptibility data and clinical effectiveness is unknown.
- † One isolate of S. equi subsp. zooepidemicus was not tested.
- ‡ The lowest MIC to encompass 50% and 90% of the most susceptible isolates, respectively.
Number of Isolates 65† 46 Time of Sample Collection Pre-Treatment Pre-Treatment MIC 50‡
(µg/mL)0.25/4.75 0.25/4.75 MIC 90‡
(µg/mL)0.25/4.75 0.25/4.75 MIC Range
(µg/mL)0.12/2.4 to 0.5/9.5 0.12/2.4 to 0.5/9.5 -
EFFECTIVENESS
A negative control, randomized, masked, field study evaluated the effectiveness of EQUISUL-SDT administered at 24 mg/kg body weight, orally, twice daily for 10 days for the treatment of lower respiratory tract infections in horses caused by Streptococcus equi subsp. zooepidemicus. In this study, a total of 182 horses were treated with EQUISUL-SDT, and 88 horses were treated with saline. One hundred seventy-three horses (112 EQUISUL-SDT and 61 saline) were included in the statistical analysis. Therapeutic success was characterized by absence of fever and no worsening of clinical signs at Day 5 and Day 10, and significant clinical improvement or resolution of clinical signs of lower respiratory tract infection by Day 17. The observed success rates are 58.9% (66/112) and 14.8% (9/61) for the EQUISUL-SDT and saline-treated groups, respectively.
Table 4 summarizes the statistical analysis results on the overall success rate.
-
ANIMAL SAFETY
In a target animal safety study, EQUISUL-SDT was administered orally to 32 healthy adult horses at 0 (0×), 24 (1×), 72 (3×), or 120 (5×) mg/kg twice daily for 30 days. Loose stool was the most common abnormal observation. Observations of loose stool (pellets with liquid or unformed/cowpile stool) occurred more often in horses treated with EQUISUL-SDT with the incidence of loose stool increasing in a dose related manner. All incidents of loose stool were self-limiting and resolved without treatment.
Horses in all EQUISUL-SDT groups demonstrated statistically significantly higher mean serum creatinine concentrations, and those in the 3× and 5× groups demonstrated statistically significantly higher mean serum albumin concentrations. Statistically higher mean neutrophil counts and mean serum gamma glutamyl transferase (GGT) activity were seen in the 1× and 5× groups. Individual animal creatinine, GGT, and albumin concentrations remained within the reference range. Individual animal elevations in absolute neutrophil counts ranged up to 7.09 × 103/mcL (reference range: 1.96-5.31 × 103/mcL).
Based upon blood concentrations obtained during the study, it was noted that the sulfadiazine and trimethoprim plasma concentrations did not increase in proportion to dose. For sulfadiazine, a 3× and 5× dose resulted in an average exposure of 2.0× and 2.6× the concentrations observed following a 1× dose. For trimethoprim, the corresponding values were 2.5× and 3.5× as compared to the 1× dose. Furthermore, marked intersubject variability, particularly with sulfadiazine, resulted in substantial overlap of individual subject blood levels across the three dosing groups.
- STORAGE CONDITIONS
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 900 mL Bottle Label
NDC: 51072-020-00
EQUISUL-SDT®
(Sulfadiazine/Trimethoprim)
Antimicrobial
Oral Suspension
400 mg/mL
Approved by FDA under NADA # 141-360
CAUTION: Federal law (USA) restricts this drug to use by or on the order of a licensed veterinarian.
For use in horses only.
900 mL
aurora pharmaceutical®

-
INGREDIENTS AND APPEARANCE
EQUISUL-SDT
sulfadiazine and trimethoprim suspensionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 51072-020 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFADIAZINE (UNII: 0N7609K889) (SULFADIAZINE - UNII:0N7609K889) SULFADIAZINE 333 mg in 1 mL TRIMETHOPRIM (UNII: AN164J8Y0X) (TRIMETHOPRIM - UNII:AN164J8Y0X) TRIMETHOPRIM 67 mg in 1 mL Product Characteristics Color WHITE Score Shape Size Flavor APPLE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51072-020-00 900 mL in 1 BOTTLE 2 NDC: 51072-020-01 135 mL in 1 BOTTLE 3 NDC: 51072-020-02 560 mL in 1 BOTTLE 4 NDC: 51072-020-03 280 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141360 08/05/2013 Labeler - Aurora Pharmaceutical, Inc. (832848639) Establishment Name Address ID/FEI Business Operations Aurora Pharmaceutical, Inc. 832848639 MANUFACTURE
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.