CYANOKIT- hydroxocobalamin injection, powder, lyophilized, for solution
Cyanokit by
Drug Labeling and Warnings
Cyanokit by is a Prescription medication manufactured, distributed, or labeled by Meridian Medical Technologies, Inc., Merck Santé s.a.s. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CYANOKIT safely and effectively. See full prescribing information for CYANOKIT.
CYANOKIT® (hydroxocobalamin for injection) for intravenous infusion
Initial U.S. Approval: 1975RECENT MAJOR CHANGES
INDICATIONS AND USAGE
CYANOKIT is indicated for the treatment of known or suspected cyanide poisoning. (1)
DOSAGE AND ADMINISTRATION
- If clinical suspicion of cyanide poisoning is high, administer CYANOKIT without delay and in conjunction with appropriate airway, ventilatory, and circulatory support, oxygen administration as well as management of seizures. (2.1)
- The expert advice of a regional poison control center may be obtained by calling 1-800-222-1222. (2.1)
Dosing:
- The starting dose of CYANOKIT for adults is 5 g, administered by intravenous infusion over 15 minutes. One 5 g vial is a complete starting dose. (2.2)
- Depending upon the severity of the poisoning and the clinical response, a second dose of 5 g may be administered by intravenous infusion for a total dose of 10 g. (2.2)
- The rate of infusion for the second 5 g dose may range from 15 minutes (for patients in extremis) to 2 hours based on patient condition. (2.2)
- The recommended diluent is 0.9% Sodium Chloride injection. (2.3)
- CYANOKIT requires a separate intravenous line for administration. (2.4)
DOSAGE FORMS AND STRENGTHS
- CYANOKIT (hydroxocobalamin for injection) for intravenous infusion consists of 1 vial, containing 5 g lyophilized hydroxocobalamin dark red crystalline powder for injection. After reconstitution, the vial contains hydroxocobalamin for injection, 25 mg/mL. (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
- Risk of Anaphylaxis and Other Hypersensitivity Reactions: Consider alternative therapies, if available, in patients with known anaphylactic reactions to hydroxocobalamin or cyanocobalamin. (5.2)
- Risk of Renal Injury: Acute renal failure with acute tubular necrosis, renal impairment and urine calcium oxalate crystals have been reported following CYANOKIT therapy. Monitor renal function for 7 days following CYANOKIT therapy. (5.3)
- Risk of Increased Blood Pressure: Substantial increases in blood pressure may occur following CYANOKIT therapy. Monitor blood pressure during treatment. (5.4)
ADVERSE REACTIONS
Most common adverse reactions (>5%) include transient chromaturia, erythema, oxalate crystals in urine, rash, increased blood pressure, nausea, headache, and infusion site reactions. (6.1)
To report SUSPECTED ADVERSE REACTIONS contact Meridian Medical Technologies®, Inc. at 1-800-438-1985, or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
2.2 Recommended Dosing
2.3 Preparation of Solution for Infusion
2.4 Incompatibility Information
2.5 Storage of Reconstituted Drug Product
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Emergency Patient Management
5.2 Risk of Anaphylactic and Other Hypersensitivity Reactions
5.3 Risk of Renal Injury
5.4 Risk of Increased Blood Pressure
5.5 Interference with Clinical Laboratory Evaluations and Clinical Methods
5.6 Photosensitivity
5.7 Use of Blood Cyanide Assay
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Animal Efficacy (Dog) Study of CYANOKIT for Cyanide Poisoning
14.2 Smoke Inhalation Victims
14.3 Cyanide Poisoning by Ingestion or Inhalation
14.4 Cross-Study Findings
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
- If clinical suspicion of cyanide poisoning is high, administer CYANOKIT without delay.
- Comprehensive treatment of acute cyanide intoxication requires support of vital functions. Airway, ventilatory and circulatory support, oxygen administration, and management of seizures should not be delayed to administer CYANOKIT [see Warnings and Precautions (5.1)].
- The expert advice of a regional poison control center may be obtained by calling 1-800-222-1222.
Identifying Patients with Cyanide Poisoning
Cyanide poisoning may result from inhalation, ingestion, or dermal exposure to various cyanide-containing compounds, including smoke from closed-space fires. Sources of cyanide poisoning include hydrogen cyanide and its salts, cyanogenic plants, aliphatic nitriles, and prolonged exposure to sodium nitroprusside.
The presence and extent of cyanide poisoning are often initially unknown. There is no widely available, rapid, confirmatory cyanide blood test. Treatment decisions must be made on the basis of clinical history and signs and symptoms of cyanide intoxication.
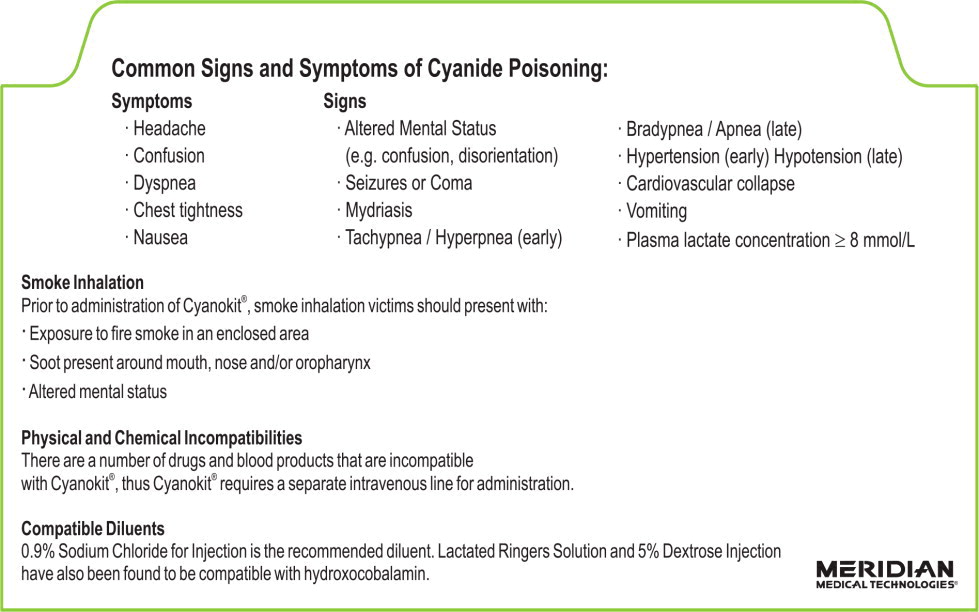
Table 1 Common Signs and Symptoms of Cyanide Poisoning Symptoms
- Headache
- Confusion
- Dyspnea
- Chest tightness
- Nausea
Signs
- Altered Mental Status (e.g., confusion, disorientation)
- Seizures or Coma
- Mydriasis
- Tachypnea / Hyperpnea (early)
- Bradypnea / Apnea (late)
- Hypertension (early) / Hypotension (late)
- Cardiovascular collapse
- Vomiting
- Plasma lactate concentration ≥8 mmol/L
In some settings, panic symptoms including tachypnea and vomiting may mimic early cyanide poisoning signs. The presence of altered mental status (e.g., confusion and disorientation) and/or mydriasis is suggestive of true cyanide poisoning although these signs can occur with other toxic exposures as well.
Smoke Inhalation
Not all smoke inhalation victims will have cyanide poisoning and may present with burns, trauma, and exposure to other toxic substances making a diagnosis of cyanide poisoning particularly difficult. Prior to administration of CYANOKIT, smoke-inhalation victims should be assessed for the following:
- Exposure to fire or smoke in an enclosed area
- Presence of soot around the mouth, nose or oropharynx
- Altered mental status
Although hypotension is highly suggestive of cyanide poisoning, it is only present in a small percentage of cyanide-poisoned smoke inhalation victims. Also indicative of cyanide poisoning is a plasma lactate concentration ≥10 mmol/L (a value higher than that typically listed in the table of signs and symptoms of isolated cyanide poisoning because carbon monoxide associated with smoke inhalation also contributes to lactic acidemia). If cyanide poisoning is suspected, treatment should not be delayed to obtain a plasma lactate concentration.
Use with Other Cyanide Antidotes
The safety of administering other cyanide antidotes simultaneously with CYANOKIT has not been established. If a decision is made to administer another cyanide antidote with CYANOKIT, these drugs should not be administered concurrently in the same intravenous line [see Dosage and Administration (2.4)].
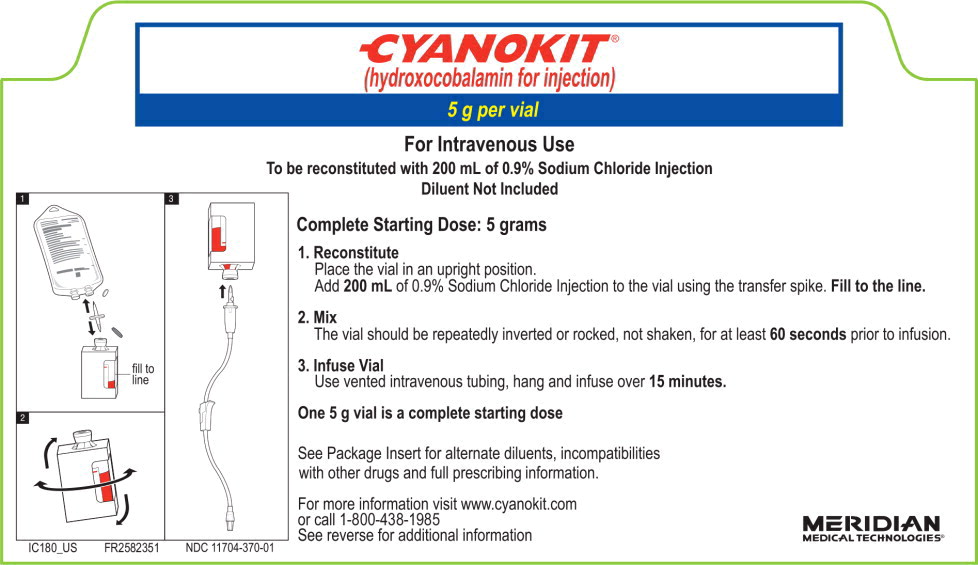
2.2 Recommended Dosing
The starting dose of hydroxocobalamin for adults is 5 g administered as an intravenous infusion over 15 minutes (approximately 15 mL/min). Administration of the entire vial constitutes a complete starting dose. Depending upon the severity of the poisoning and the clinical response, a second dose of 5 g may be administered by intravenous infusion for a total dose of 10 g. The rate of infusion for the second dose may range from 15 minutes (for patients in extremis) to two hours, as clinically indicated.
2.3 Preparation of Solution for Infusion
Reconstitute the 5 g vial of hydroxocobalamin with 200 mL of diluent (not provided with CYANOKIT) using the supplied sterile transfer spike. The recommended diluent is 0.9% Sodium Chloride injection (0.9% NaCl). Lactated Ringers injection and 5% Dextrose injection (D5W) have also been found to be compatible with hydroxocobalamin and may be used if 0.9% NaCl is not readily available. The line on the vial label represents 200 mL volume of diluent. Following the addition of diluent to the lyophilized powder, the vial should be repeatedly inverted or rocked, not shaken, for at least 60 seconds prior to infusion.
Visually inspect hydroxocobalamin solutions for particulate matter and color prior to administration. If the reconstituted solution is not dark red or if particulate matter is observed after the solution has been appropriately mixed, the solution should be discarded.
2.4 Incompatibility Information
Physical incompatibility (particle formation) and chemical incompatibility were observed with the mixture of hydroxocobalamin in solution with selected drugs that are frequently used in resuscitation efforts. Hydroxocobalamin is also chemically incompatible with sodium thiosulfate and sodium nitrite and has been reported to be incompatible with ascorbic acid. Therefore, these and other drugs should not be administered simultaneously through the same intravenous line as hydroxocobalamin.
Simultaneous administration of hydroxocobalamin and blood products (whole blood, packed red cells, platelet concentrate and/or fresh frozen plasma) through the same intravenous line is not recommended. However, blood products and hydroxocobalamin can be administered simultaneously using separate intravenous lines (preferably on contralateral extremities, if peripheral lines are being used).
-
3 DOSAGE FORMS AND STRENGTHS
CYANOKIT (hydroxocobalamin for injection) for intravenous infusion consists of 1 vial, containing 5 g lyophilized hydroxocobalamin dark red crystalline powder for injection. After reconstitution, the vial contains hydroxocobalamin for injection, 25 mg/mL [see How Supplied/Storage and Handling (16) for full kit description].
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Emergency Patient Management
In conjunction with CYANOKIT, treatment of cyanide poisoning must include immediate attention to airway patency, adequacy of oxygenation and hydration, cardiovascular support, and management of seizures. Consideration should be given to decontamination measures based on the route of exposure.
5.2 Risk of Anaphylactic and Other Hypersensitivity Reactions
Use caution in the management of patients with known anaphylactic reactions to hydroxocobalamin or cyanocobalamin. Consider alternative therapies, if available.
Allergic reactions may include: anaphylaxis, chest tightness, edema, urticaria, pruritus, dyspnea, and rash.
Allergic reactions including angioneurotic edema have also been reported in postmarketing experience.
5.3 Risk of Renal Injury
Cases of acute renal failure with acute tubular necrosis, renal impairment, and urine calcium oxalate crystals have been reported. In some situations, hemodialysis was required to achieve recovery. Regular monitoring of renal function, including but not limited to blood urea nitrogen (BUN) and serum creatinine, should be performed for 7 days following CYANOKIT therapy.
5.4 Risk of Increased Blood Pressure
Many patients with cyanide poisoning will be hypotensive; however, elevations in blood pressure have also been observed in known or suspected cyanide poisoning victims.
Elevations in blood pressure (≥180 mmHg systolic or ≥110 mmHg diastolic) were observed in approximately 18% of healthy subjects (not exposed to cyanide) receiving hydroxocobalamin 5 g and 28% of subjects receiving 10 g. Increases in blood pressure were noted shortly after the infusions were started; the maximal increase in blood pressure was observed toward the end of the infusion. These elevations were generally transient and returned to baseline levels within 4 hours of dosing. Monitor blood pressure during treatment with CYANOKIT.
5.5 Interference with Clinical Laboratory Evaluations and Clinical Methods
Clinical Laboratory Evaluations
Because of its deep red color, hydroxocobalamin has been found to interfere with colorimetric determination of certain laboratory parameters (e.g., clinical chemistry, hematology, coagulation, and urine parameters). In vitro tests indicated that the extent and duration of the interference are dependent on numerous factors such as the dose of hydroxocobalamin, analyte, methodology, analyzer, hydroxocobalamin concentration, and partially on the time between sampling and measurement.
The data presented in Table 2 is collected from in vitro studies and pharmacokinetic data in healthy volunteers and describes laboratory interference that may be observed following a 5 g dose of hydroxocobalamin. Interference following a 10 g dose can be expected to last up to an additional 24 hours. The extent and duration of interference in cyanide-poisoned patients may differ. In addition, results may vary substantially from one analyzer to another. Be aware of this when reporting and interpreting laboratory results.
Table 2 Laboratory Interference Observed with in vitro Samples of Hydroxocobalamin Laboratory Parameter No Interference Observed Artificially Increased * Artificially Decreased * Un-predictable Duration of Interference * ≥10% interference observed on at least 1 analyzer
Analyzers used: ACL Futura (Instrumentation Laboratory), AxSYM®/Architect™ (Abbott), BM Coasys110 (Boehringer Mannheim), CellDyn 3700® (Abbott), Clinitek® 500 (Bayer), Cobas Integra® 700, 400 (Roche), Gen-S Coultronics, Hitachi 917, STA® Compact, Vitros® 950 (Ortho Diagnostics)
Clinical Chemistry Calcium
Sodium
Potassium
Chloride
Urea
GGTCreatinine
Bilirubin
Triglycerides
Cholesterol
Total protein
Glucose
Albumin
Alkaline phosphataseALT
AmylasePhosphate
Uric Acid
AST
CK
CKMB
LDH24 hours with the exception of bilirubin (up to 4 days) Hematology Erythrocytes
Hematocrit
MCV
Leukocytes
Lymphocytes
Monocytes
Eosinophils
Neutrophils
PlateletsHemoglobin
MCH
MCHC
Basophils12 - 16 hours Coagulation aPTT
PT (Quick or INR)24 - 48 hours Urinalysis pH (with all doses)
Glucose
Protein
Erythrocytes
Leukocytes
Ketones
Bilirubin
Urobilinogen
NitritepH (with equivalent doses of <5 g) 48 hours up to 8 days; color changes may persist up to 28 days 5.6 Photosensitivity
Hydroxocobalamin absorbs visible light in the UV spectrum. It therefore has potential to cause photosensitivity. While it is not known if the skin redness predisposes to photosensitivity, patients should be advised to avoid direct sun while their skin remains discolored.
5.7 Use of Blood Cyanide Assay
While determination of blood cyanide concentration is not required for management of cyanide poisoning and should not delay treatment with CYANOKIT, collecting a pretreatment blood sample may be useful for documenting cyanide poisoning as sampling post-CYANOKIT use may be inaccurate.
-
6 ADVERSE REACTIONS
Serious adverse reactions with hydroxocobalamin include allergic reactions, renal injury, and increases in blood pressure [see Warnings and Precautions (5.2, 5.3, 5.4)].
6.1 Clinical Studies Experience
Experience in Healthy Subjects
Because clinical trials were conducted under widely varying conditions, adverse reaction rates observed in the clinical trials may not reflect the rates observed in practice.
A double-blind, randomized, placebo-controlled, single-ascending-dose (2.5, 5, 7.5, and 10 g) study was conducted to assess the safety, tolerability, and pharmacokinetics of hydroxocobalamin in 136 healthy adult subjects. Because of the dark red color of hydroxocobalamin, the two most frequently occurring adverse reactions were chromaturia (red-colored urine) which was reported in all subjects receiving a 5 g dose or greater; and erythema (skin redness), which occurred in most subjects receiving a 5 g dose or greater. Adverse reactions reported in at least 5% of the 5 g dose group and corresponding rates in the 10 g and placebo groups are shown in Table 3.
Table 3 Incidence of Adverse Reactions Occurring in >5% of Subjects in 5 g Dose Group and Corresponding Incidence in 10 g Dose Group and Placebo * Rashes were predominantly acneiform
ADR 5 g Dose Group 10 g Dose Group Hydroxocobalamin
N=66
n (%)Placebo
N=22
n (%)Hydroxocobalamin
N=18
n (%)Placebo
N=6
n (%)Chromaturia (red colored urine) 66 (100) 0 18 (100) 0 Erythema 62 (94) 0 18 (100) 0 Oxalate crystals in urine 40 (61) 1 (5) 10 (56) 0 Rash* 13 (20) 0 8 (44) 0 Blood pressure increased 12 (18) 0 5 (28) 0 Nausea 4 (6) 1 (5) 2 (11) 0 Headache 4 (6) 1 (5) 6 (33) 0 Lymphocyte percent decreased 5 (8) 0 3 (17) 0 Infusion site reaction 4 (6) 0 7 (39) 0 In this study, the following adverse reactions were reported to have occurred in a dose-dependent fashion and with greater frequency than observed in placebo-treated cohorts: increased blood pressure (particularly diastolic blood pressure), rash, nausea, headache and infusion site reactions. All were mild to moderate in severity and resolved spontaneously when the infusion was terminated or with standard supportive therapies.
Other adverse reactions reported in this study and considered clinically relevant were:
- Eye disorders: swelling, irritation, redness
- Gastrointestinal disorders: dysphagia, abdominal discomfort, vomiting, diarrhea, dyspepsia, hematochezia
- General disorders and administration site conditions: peripheral edema, chest discomfort
- Immune system disorders: allergic reaction
- Nervous system disorders: memory impairment, dizziness
- Psychiatric disorders: restlessness
- Respiratory, thoracic and mediastinal disorders: dyspnea, throat tightness, dry throat
- Skin and subcutaneous tissue disorders: urticaria, pruritus
- Vascular disorders: hot flush
Experience in Known or Suspected Cyanide Poisoning Victims
Four open-label, uncontrolled, clinical studies (one of which was prospective and three of which were retrospective) were conducted in known or suspected cyanide-poisoning victims. A total of 245 patients received hydroxocobalamin treatment in these studies. Systematic collection of adverse events was not done in all of these studies and interpretation of causality is limited due to the lack of a control group and due to circumstances of administration (e.g., use in fire victims). Adverse reactions reported in these studies listed by system organ class included:
- Cardiac disorders: ventricular extrasystoles
- Investigations: electrocardiogram repolarization abnormality, heart rate increased
- Respiratory, thoracic, and mediastinal disorders: pleural effusion
Adverse reactions common to both the studies in known or suspected cyanide poisoning victims and the study in healthy volunteers are listed in the healthy volunteer section only and are not duplicated in this list.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of CYANOKIT. Because adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency.
Cases of acute renal failure with acute tubular necrosis, renal impairment, and urine calcium oxalate crystals have been reported in patients treated with CYANOKIT.
-
7 DRUG INTERACTIONS
Formal drug interaction studies have not been conducted with CYANOKIT.
Interference with Laboratory Tests
Because of its deep red color, hydroxocobalamin has been found to interfere with colorimetric determination of certain laboratory parameters (e.g., clinical chemistry, hematology, coagulation, and urine parameters). Be aware of this when reporting and interpreting laboratory results [see Warnings and Precautions (5.5)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from cases reported in the published literature and postmarketing surveillance with CYANOKIT use in pregnant women are insufficient to identify a drug-associated risk for major birth defects, miscarriage, or adverse maternal and fetal outcomes. There are risks to the pregnant woman and fetus associated with untreated cyanide poisoning (see Clinical Considerations). In animal studies, hydroxocobalamin administered to pregnant rats and rabbits during the period of organogenesis caused skeletal and soft tissue abnormalities, including alterations in the central nervous system, at exposures similar to human exposures at the therapeutic dose (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
In animal studies, pregnant rats and rabbits received CYANOKIT (75, 150, or 300 mg/kg/d) during the period of organogenesis. Following intraperitoneal dosing in rats and intravenous dosing in rabbits, maternal exposures were equivalent to 0.5, 1, or 2 times the human exposure at the therapeutic dose (based on AUC). In the high dose groups for both species, maternal toxicity occurred, and there was a reduced number of live fetuses due to embryofetal resorptions. In addition, decreased live fetal weight occurred in high dose rats, but not in rabbits. Incomplete skeletal ossification occurred in both rats and rabbits. In rats, two fetuses of the high dose group and two fetuses of the mid dose group (each from a different litter) had short, rudimentary or small front or hind legs. Rabbit litters and fetuses exhibited a dose-dependent increase in various gross soft tissue and skeletal anomalies. The main findings in rabbits were flexed, rigid flexor or medially rotated forelimbs or hindlimbs and domed heads at external examination; enlarged anterior or posterior fontanelles of the ventricles of the brain and flat, bowed or large ribs at skeletal examination; and dilated ventricles of the brain, and thick wall of the stomach at visceral examination. It is unknown if similar findings would be observed in rats and rabbits if CYANOKIT was administered as a single dose during any critical period of development.
8.2 Lactation
Risk Summary
Breastfeeding is not recommended during treatment with CYANOKIT. There are no data to determine when breastfeeding may be safely restarted following administration of CYANOKIT. Hydroxocobalamin and Vitamin B12 (which is formed when hydroxocobalamin combines with cyanide) are present in human milk. There are no data on the effects of hydroxocobalamin on the breastfed infant or the effects on milk production.
8.4 Pediatric Use
Safety and effectiveness of CYANOKIT have not been established in this population. In non-US marketing experience, a dose of 70 mg/kg has been used to treat pediatric patients.
8.5 Geriatric Use
Approximately 50 known or suspected cyanide poisoning victims aged 65 or older received hydroxocobalamin in clinical studies. In general, the safety and effectiveness of hydroxocobalamin in these patients was similar to that of younger patients. No adjustment of dose is required in elderly patients.
-
10 OVERDOSAGE
No data are available about overdose with CYANOKIT in adults. Should overdose occur, treatment should be directed to the management of symptoms. Hemodialysis may be effective in such a circumstance, but is only indicated in the event of significant hydroxocobalamin-related toxicity. Because of its deep red color, hydroxocobalamin may interfere with the performance of hemodialysis machines [see Warnings and Precautions (5.5)].
-
11 DESCRIPTION
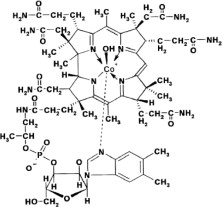
Hydroxocobalamin, the active ingredient in CYANOKIT, is cobinamide dihydroxide dihydrogen phosphate (ester), mono (inner salt), 3'-ester with 5,6-dimethyl-1-α-D-ribofuranosyl-1H-benzimidazole, an antidote. The drug substance is the hydroxylated active form of vitamin B12 and is a large molecule in which a trivalent cobalt ion is coordinated in four positions by a tetrapyrol (or corrin) ring. It is a hygroscopic, odorless, dark red, crystalline powder that is freely soluble in water and ethanol, and practically insoluble in acetone and diethyl ether. Hydroxocobalamin has a molecular weight of 1346.36 atomic mass units, an empirical formula of C62H89CoN13O15P and the following structural formula:
CYANOKIT (hydroxocobalamin for injection) for intravenous infusion is a cyanide antidote package which contains one colorless 250 mL glass vial, containing 5 g dark red lyophilized hydroxocobalamin, pH adjusted with hydrochloric acid, one transfer spike, one intravenous administration set, one quick use reference guide and one package insert.
The 5 g vial of hydroxocobalamin for injection is to be reconstituted with 200 mL of 0.9% NaCl, to give a dark red injectable solution (25 mg/mL). If 0.9% NaCl is not readily available, 200 mL of either Lactated Ringers injection or 5% Dextrose injection (D5W) may be used as the diluent. Diluent is not included in the CYANOKIT. The pH of the reconstituted product ranges from 3.5 to 6.0.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Cyanide is an extremely toxic poison. In the absence of rapid and adequate treatment, exposure to a high dose of cyanide can result in death within minutes due to the inhibition of cytochrome oxidase resulting in arrest of cellular respiration. Specifically, cyanide binds rapidly with cytochrome a3, a component of the cytochrome c oxidase complex in mitochondria. Inhibition of cytochrome a3 prevents the cell from using oxygen and forces anaerobic metabolism, resulting in lactate production, cellular hypoxia and metabolic acidosis. In massive acute cyanide poisoning, the mechanism of toxicity may involve other enzyme systems as well. Signs and symptoms of acute systemic cyanide poisoning may develop rapidly within minutes, depending on the route and extent of cyanide exposure.
The action of CYANOKIT in the treatment of cyanide poisoning is based on its ability to bind cyanide ions. Each hydroxocobalamin molecule can bind one cyanide ion by substituting it for the hydroxo ligand linked to the trivalent cobalt ion, to form cyanocobalamin, which is then excreted in the urine.
12.2 Pharmacodynamics
Administration of CYANOKIT to cyanide-poisoned patients with the attendant formation of cyanocobalamin resulted in increases in blood pressure and variable changes in heart rate upon initiation of hydroxocobalamin infusions [see Warnings and Precautions (5.4)].
12.3 Pharmacokinetics
Following intravenous administration of hydroxocobalamin significant binding to plasma proteins and low molecular weight physiological compounds occurs, forming various cobalamin-(III) complexes by replacing the hydroxo ligand. The low molecular weight cobalamins-(III) formed, including hydroxocobalamin, are termed “free cobalamins-(III)”; the sum of free and protein-bound cobalamins is termed “total cobalamins-(III)”. In order to reflect the exposure to the sum of all derivatives, pharmacokinetics of cobalamins-(III) (i.e., cobalamin-(III) entity without specific ligand) were investigated instead of hydroxocobalamin alone, using the concentration unit μg eq/mL.
Dose-proportional pharmacokinetics was observed following single dose intravenous administration of 2.5 to 10 g of hydroxocobalamin in healthy volunteers. Mean free and total cobalamins-(III) Cmax values of 113 and 579 μg eq/mL, respectively, were determined following a dose of 5 g of hydroxocobalamin. Similarly, mean free and total cobalamins-(III) Cmax values of 197 and 995 μg eq/mL, respectively, were determined following the dose of 10 g of hydroxocobalamin.
When normalized for body weight, male and female subjects revealed no major differences in pharmacokinetic parameters of free and total cobalamins-(III) following the administration of 5 and 10 g of hydroxocobalamin.
Distribution
The volume of distribution at steady state (Vss) for both free and total cobalamins-(III) showed no apparent relationship to dose. The Vss ranged from 280.7 to 349.5 L for free cobalamins-(III), and from 21.8 to 25.6 L for total cobalamins-(III). The comparatively high values for Vss of free cobalamins-(III) are due to the high protein binding of hydroxocobalamin as it reacts in the blood with plasma constituents to form cobalamins-(III) complexes and the rapid distribution of free cobalamins-(III) into tissues.
Elimination
The mean total amount of cobalamins-(III) excreted in urine during the collection period of 72 hours was about 60% of a 5 g dose and about 50% of a 10 g dose of hydroxocobalamin. Overall, the total urinary excretion was calculated to be at least 60 to 70% of the administered dose. The majority of the urinary excretion occurred during the first 24 hours, but red-colored urine was observed for up to 35 days following the intravenous infusion. The mean half-life of free and total cobalamins-(III) was found to be approximately 26 to 31 hours at both the 5 g and 10 g dose level.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term animal studies have not been performed to evaluate the carcinogenic potential of hydroxocobalamin.
-
14 CLINICAL STUDIES
14.1 Animal Efficacy (Dog) Study of CYANOKIT for Cyanide Poisoning
The effectiveness of CYANOKIT for treatment of cyanide poisoning has not been determined in humans because inducing cyanide poisoning in humans to study the drug's efficacy is not ethical. Therefore, the effectiveness of CYANOKIT for cyanide poisoning was established based on the results of the adequate and well-controlled animal efficacy study described below. While the results of this animal study cannot be extrapolated to humans with certainty, the extrapolation is supported by the understanding of the pathophysiologic mechanisms of the toxicity of cyanide and the mechanisms of the protective effect of hydroxocobalamin as examined in dogs. In addition, the results of uncontrolled human studies and the animal study establish that hydroxocobalamin is likely to produce clinical benefit in humans.
The effectiveness of hydroxocobalamin was examined in a randomized, placebo-controlled, blinded study in cyanide-poisoned adult dogs assigned to treatment with vehicle (0.9% saline), or 75 or 150 mg/kg hydroxocobalamin. Anesthetized dogs were poisoned by intravenous administration of a lethal dose of potassium cyanide. Dogs then received vehicle or 75 or 150 mg/kg hydroxocobalamin, administered intravenously over 7.5 minutes. The 75 and 150 mg/kg doses are approximately equivalent to 5 and 10 g of hydroxocobalamin (respectively) in humans based on both body weight and the Cmax of hydroxocobalamin (total cobalamins-(III)). Survival at 4 hours and at 14 days was significantly greater in low-and high-dose groups compared with dogs receiving vehicle alone (Table 4). Hydroxocobalamin reduced whole blood cyanide concentrations by approximately 50% by the end of the infusion compared with vehicle.
Table 4 Survival of Cyanide-Poisoned Dogs Parameter
Treatment Vehicle
N=17CYANOKIT 75 mg/kg
N=19150 mg/kg
N=18Survival at Hour 4, n (%) 7 (41) 18 (95) 18 (100) Survival at Day 14, n (%) 3 (18) 15 (79) 18 (100) Histopathology revealed brain lesions that were consistent with cyanide-induced hypoxia. The incidence of brain lesions was markedly lower in hydroxocobalamin treated animals compared to vehicle treated groups.
14.2 Smoke Inhalation Victims
A prospective, uncontrolled, open-label study was carried out in 69 subjects who had been exposed to smoke inhalation from fires. Subjects had to be over 15 years of age, present with soot in the mouth and expectoration (to indicate significant smoke exposure), and have altered neurological status. The median hydroxocobalamin dose was 5 g with a range from 4 to 15 g.
Fifty of 69 subjects (73%) survived following treatment with hydroxocobalamin. Nineteen subjects treated with hydroxocobalamin did not survive. Fifteen patients treated with hydroxocobalamin were in cardiac arrest initially at the scene; 13 of these subjects died and 2 survived.
Of the 42 subjects with pretreatment cyanide levels considered to be potentially toxic, 28 (67%) survived. Of the 19 subjects whose pretreatment cyanide levels were considered potentially lethal, 11 (58%) survived. Of the 50 subjects who survived, 9 subjects (18%) had neurological sequelae at hospital discharge. These included dementia, confusion, psychomotor retardation, anterograde amnesia, intellectual deterioration moderate cerebellar syndrome, aphasia, and memory impairment.
Two additional retrospective, uncontrolled studies were carried out in subjects who had been exposed to cyanide from fire or smoke inhalation. Subjects were treated with up to 15 g of hydroxocobalamin. Survival in these two studies was 34 of 61 (56%) for one study, and 30 of 72 (42%) for the second.
14.3 Cyanide Poisoning by Ingestion or Inhalation
A retrospective, uncontrolled study was carried out in 14 subjects who had been exposed to cyanide from sources other than from fire or smoke (i.e., ingestion or inhalation). Subjects were treated with 5 to 20 g of hydroxocobalamin. Eleven of 12 subjects whose blood cyanide concentration was known had initial blood cyanide levels considered to be above the lethal threshold.
Ten of 14 subjects (71%) survived, following administration of hydroxocobalamin. One of the four subjects who died had presented in cardiac arrest. Of the 10 subjects who survived, only 1 subject had neurological sequelae at hospital discharge. This subject had post-anoxic encephalopathy, with memory impairment, considered to be due to cyanide poisoning.
14.4 Cross-Study Findings
Experience with Dosing Greater than 10 g of Hydroxocobalamin
Across all four uncontrolled studies, 10 patients who did not demonstrate a full response to 5 or 10 g-doses of hydroxocobalamin were treated with more than 10 g of hydroxocobalamin. One of these 10 patients survived with unspecified neurological sequelae.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Each CYANOKIT carton (NDC: 11704-370-01) consists of the following:
- One 250 mL glass vial, containing lyophilized hydroxocobalamin for injection, 5 g
- One sterile transfer spike
- One sterile intravenous infusion set
- One quick use reference guide
- One package insert
Diluent is not included.
Storage
Lyophilized form
Store at 25°C (77°F); excursions permitted to 15-30°C (59 to 86°F) [see USP Controlled Room Temperature].
CYANOKIT may be exposed during short periods to the temperature variations of usual transport (15 days submitted to temperatures ranging from 5 to 40°C (41 to 104°F), transport in the desert (4 days submitted to temperatures ranging from 5 to 60°C (41 to 140°F)) and freezing/defrosting cycles (15 days submitted to temperatures ranging from -20 to 40°C (-4 to 104°F)).
-
17 PATIENT COUNSELING INFORMATION
CYANOKIT is indicated for cyanide poisoning and in this setting, patients will likely be unresponsive or may have difficulty in comprehending counseling information.
Erythema and Chromaturia
Advise patients that skin redness may last up to 2 weeks and urine coloration may last for up to 5 weeks after administration of CYANOKIT. While it is not known if the skin redness predisposes to photosensitivity, patients should be advised to avoid direct sun while their skin remains discolored.
Rash
Inform patients that an acneiform rash may appear anywhere from 7 to 28 days following hydroxocobalamin treatment. This rash will usually resolve without treatment within a few weeks.
Renal Function Monitoring
Advise patients that renal function will be monitored for 7 days following treatment with CYANOKIT or, in the event of renal impairment, until renal function returns to normal.
Pregnancy
Advise pregnant women that maternal cyanide poisoning results in fetal cyanide poisoning. Treatment for cyanide poisoning may be lifesaving for both the pregnant woman and fetus. Advise females of reproductive potential to notify their provider if they were pregnant during therapy with CYANOKIT [see USE IN SPECIFIC POPULATIONS (8.1)].
Lactation
Advise women that breastfeeding is not recommended during treatment with CYANOKIT [see USE IN SPECIFIC POPULATIONS (8.2)].
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 12/2018
Patient Information
CYANOKIT (hydroxocobalamin for injection)
for intravenous infusionWhat is CYANOKIT?
CYANOKIT is prescription medicine used for the treatment of known or suspected cyanide poisoning. Cyanide is a chemical poison. Cyanide poisoning can happen from:
breathing smoke from household and industrial fires
breathing or swallowing cyanide
having your skin exposed to cyanide
The effectiveness of CYANOKIT was based on a study in animals, because intentionally exposing humans to cyanide is not ethical. The safety of CYANOKIT was studied in animals and healthy people and derived from experience in patients exposed to cyanide.
It is not known if CYANOKIT is safe and effective in children.
Cyanide poisoning is a life-threatening condition because cyanide stops your body from being able to use oxygen. You can die if your body does not have enough oxygen.Tell your healthcare provider if you:
- have had an allergic reaction to hydroxocobalamin or cyanocobalamin
- are pregnant or think you may have been pregnant during treatment with CYANOKIT. CYANOKIT may harm your unborn baby. However, treatment for cyanide poisoning may save your life and the life of your unborn baby.
-
are breastfeeding. Breastfeeding is not recommended during treatment with CYANOKIT. Talk to your healthcare provider about the best way to feed your baby during this time.
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. How is CYANOKIT given?
- Your healthcare provider will give you CYANOKIT through a vein by intravenous (IV) infusion over 15 minutes.
- A second dose of CYANOKIT may be given to you if needed.
What should I avoid after I receive CYANOKIT?
- CYANOKIT may cause red colored skin. Skin redness is common during treatment with CYANOKIT and may last up to 2 weeks after treatment with CYANOKIT. You should avoid sunlight while your skin is red.
What are the possible side effects of CYANOKIT?
CYANOKIT may cause serious side effects, including:
- Allergic reactions. Signs and symptoms of a serious allergic reaction include chest tightness, trouble breathing, swelling, hives, itching, and rash. Seek emergency help if you experience signs and/or symptoms of an allergic reaction.
- Kidney problems. CYANOKIT can cause kidney problems, including kidney failure. Tell your healthcare provider if you develop crystals in your urine. Your healthcare provider will monitor your kidney function for 7 days after treatment with CYANOKIT, or longer if needed.
-
Increased blood pressure. Increased blood pressure is a common but serious side effect during treatment with CYANOKIT. Your healthcare provider will monitor your blood pressure during treatment with CYANOKIT.
The most common side effects of CYANOKIT include:
- red colored urine. Red colored urine redness may last up to 5 weeks after treatment with CYANOKIT.
- nausea
- headache
- reactions at the site of infusion
- acne-like rash. Acne-like rash may appear 7 to 28 days after treatment with CYANOKIT. This rash usually goes away without any treatment.
These are not all the side effects with CYANOKIT.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.General information about the safe and effective use of CYANOKIT.
This Patient Information leaflet summarizes the most important information about CYANOKIT. If you would like more information, talk to your healthcare provider. You can ask your pharmacist or healthcare provider for information about CYANOKIT that is written for health professionals.What are the ingredients in CYANOKIT?
Active ingredient: hydroxocobalamin
Manufactured by: Merck Santé s.a.s., Semoy, France
Distributed by Meridian Medical Technologies®, Inc. Columbia, MD 21046
1-800-438-1985
180_US_20181_NO
For more information, go to www.CYANOKIT.com. -
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – 5 g VIAL LABEL
CYANOKIT ®
(hydroxocobalamin for Injection)
5 g per vial
For Intravenous Use
To be reconstituted with 200 mL of 0.9% Sodium Chloride Injection
Diluent Not Included
Rx Only
Manufactured by:
Merck Santé s.a.s.
Semoy, France
Distributed by
Meridian Medical Technologies®, Inc.
Columbia, MD 21046
1-800-438-1985
MERIDIAN
MEDICAL TECHNOLOGIES®
- PRINCIPAL DISPLAY PANEL
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – 5 g OUTER CARTON
NDC: 11704-370-01
CYANOKIT ®
(hydroxocobalamin for injection)
5 g per vial
For Intravenous Use
To be reconstituted with 200 mL of Sodium Chloride Injection
Diluent Not Included
Kit Contains:
1 Vial, containing Hydroxocobalamin for Injection, 5 g
1 Intravenous administration set
1 Transfer spike
1 Quick Use Reference Guide
1 Package Insert
MERIDIAN
MEDICAL TECHNOLOGIES®
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CYANOKIT
hydroxocobalamin injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 11704-370 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Hydroxocobalamin (UNII: Q40X8H422O) (Hydroxocobalamin - UNII:Q40X8H422O) Hydroxocobalamin 5 g in 250 mL Inactive Ingredients Ingredient Name Strength hydrochloric acid (UNII: QTT17582CB) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 11704-370-01 1 in 1 CARTON 10/01/2011 1 250 mL in 1 VIAL, GLASS; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022041 10/01/2011 Labeler - Meridian Medical Technologies, Inc. (167671341) Establishment Name Address ID/FEI Business Operations Merck Santé s.a.s 384668112 MANUFACTURE(11704-370) , PACK(11704-370) , LABEL(11704-370) , ANALYSIS(11704-370) Establishment Name Address ID/FEI Business Operations Meridian Medical Technologies, Inc. 167671341 LABEL(11704-370)
Trademark Results [Cyanokit]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 CYANOKIT 76130880 2697031 Live/Registered |
SERB 2000-09-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.