ULTIMATE MIRACLE WORKER MULTI-REJUVENATING SPF 30- avobenzone, octinoxate, octocrylene cream
ultimate miracle worker multi-rejuvenating spf 30 by
Drug Labeling and Warnings
ultimate miracle worker multi-rejuvenating spf 30 by is a Otc medication manufactured, distributed, or labeled by Philosophy, Inc., Process Technologies and Packaging Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
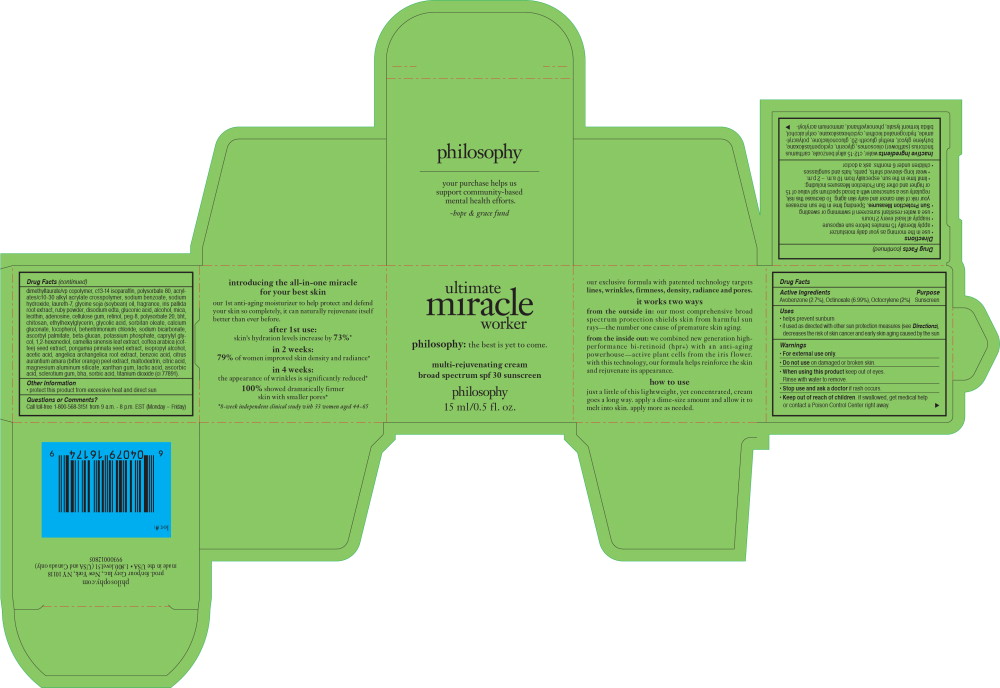
- Active Ingredients
- Purpose
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
- use in the morning as your daily moisturizer
- apply liberally 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water-resistant sunscreen if swimming or sweating
-
Sun-Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum spf value of 15 or higher and other Sun-Protection Measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months: ask a doctor
-
Inactive Ingredients
water, c12-15 alkyl benzoate, carthamus tinctorius (safflower) oleosomes, glycerin, cyclopentasiloxane, butylene glycol, methyl gluceth-20, gluconolactone, polyacrylamide, hydrogenated lecithin, cyclohexasiloxane, cetyl alcohol, bifida ferment lysate, phenoxyethanol, ammonium acryloyldimethyltaurate/vp copolymer, c13-14 isoparaffin, polysorbate 80, acrylates/c10-30 alkyl acrylate crosspolymer, sodium benzoate, sodium hydroxide, laureth-7, glycine soja (soybean) oil, fragrance, iris pallida root extract, ruby powder, disodium edta, gluconic acid, alcohol, mica, lecithin, adenosine, cellulose gum, retinol, peg-8, polysorbate 20, bht, titanium dioxide, chitosan, ethylhexylglycerin, glycolic acid, sorbitan oleate, calcium gluconate, tocopherol, behentrimoniumchloride, sodium bicarbonate, ascorbyl palmitate, beta-glucan, potassium phosphate, caprylyl glycol, 1,2-hexanediol, camellia sinensis leaf extract, coffea arabica (coffee) seed extract, pongamia pinnata seed extract, isopropyl alcohol, acetic acid, angelica archangelica root extract, benzoic acid, citrus aurantiumamara (bitter orange) peel extract, maltodextrin, citric acid, magnesium aluminum silicate, xanthan gum, lactic acid, ascorbic acid, sclerotium gum, bha, sorbic acid.
- Other Information
- Questions or Comments?
- Principal Display Panel - Ultimate Miracle Worker SPF 30 7 ml Carton Label
- Principal Display Panel - Ultimate Miracle Worker SPF 30 15 ml Carton Label
- Principal Display Panel - Ultimate Miracle Worker SPF 30 60 ml Carton Label
-
INGREDIENTS AND APPEARANCE
ULTIMATE MIRACLE WORKER MULTI-REJUVENATING SPF 30
avobenzone, octinoxate, octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 50184-1013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.7 g in 100 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 6.99 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 2 g in 100 mL Inactive Ingredients Ingredient Name Strength CALCIUM GLUCONATE (UNII: SQE6VB453K) TOCOPHEROL (UNII: R0ZB2556P8) BEHENTRIMONIUM CHLORIDE (UNII: X7GNG3S47T) SODIUM BICARBONATE (UNII: 8MDF5V39QO) ASCORBYL PALMITATE (UNII: QN83US2B0N) POTASSIUM PHOSPHATE, UNSPECIFIED FORM (UNII: B7862WZ632) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CETYL GLYCOL (UNII: 1H07JK4G0C) GREEN TEA LEAF (UNII: W2ZU1RY8B0) MILLETTIA PINNATA SEED (UNII: C2BRV53B1V) ISOPROPYL ALCOHOL (UNII: ND2M416302) ACETIC ACID (UNII: Q40Q9N063P) CITRUS AURANTIUM FRUIT RIND (UNII: 055456JHI7) ANGELICA ARCHANGELICA ROOT (UNII: DTN01M69SN) BENZOIC ACID (UNII: 8SKN0B0MIM) MALTODEXTRIN (UNII: 7CVR7L4A2D) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) XANTHAN GUM (UNII: TTV12P4NEE) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) ASCORBIC ACID (UNII: PQ6CK8PD0R) BETASIZOFIRAN (UNII: 2X51AD1X3T) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) SORBIC ACID (UNII: X045WJ989B) WATER (UNII: 059QF0KO0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CARTHAMUS TINCTORIUS SEED OLEOSOMES (UNII: 9S60Q72309) GLYCERIN (UNII: PDC6A3C0OX) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) METHYL GLUCETH-20 (UNII: J3QD0LD11P) GLUCONOLACTONE (UNII: WQ29KQ9POT) POLYACRYLAMIDE (10000 MW) (UNII: E2KR9C9V2I) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) CYCLOMETHICONE 6 (UNII: XHK3U310BA) CETYL ALCOHOL (UNII: 936JST6JCN) PHENOXYETHANOL (UNII: HIE492ZZ3T) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM HYDROXIDE (UNII: 55X04QC32I) LAURETH-7 (UNII: Z95S6G8201) SOYBEAN OIL (UNII: 241ATL177A) IRIS PALLIDA ROOT (UNII: LVV57EM5OG) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) GLUCONIC ACID (UNII: R4R8J0Q44B) ALCOHOL (UNII: 3K9958V90M) MICA (UNII: V8A1AW0880) ADENOSINE (UNII: K72T3FS567) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) RETINOL (UNII: G2SH0XKK91) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYSORBATE 20 (UNII: 7T1F30V5YH) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLIGLUSAM (UNII: 82LKS4QV2Y) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCOLIC ACID (UNII: 0WT12SX38S) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50184-1013-1 1 in 1 CARTON 04/01/2018 1 7 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 50184-1013-2 1 in 1 CARTON 04/01/2018 2 15 mL in 1 JAR; Type 0: Not a Combination Product 3 NDC: 50184-1013-3 1 in 1 CARTON 04/01/2018 3 60 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 04/01/2018 Labeler - Philosophy, Inc. (948102256) Establishment Name Address ID/FEI Business Operations Process Technologies and Packaging Inc. 809172885 manufacture(50184-1013)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.