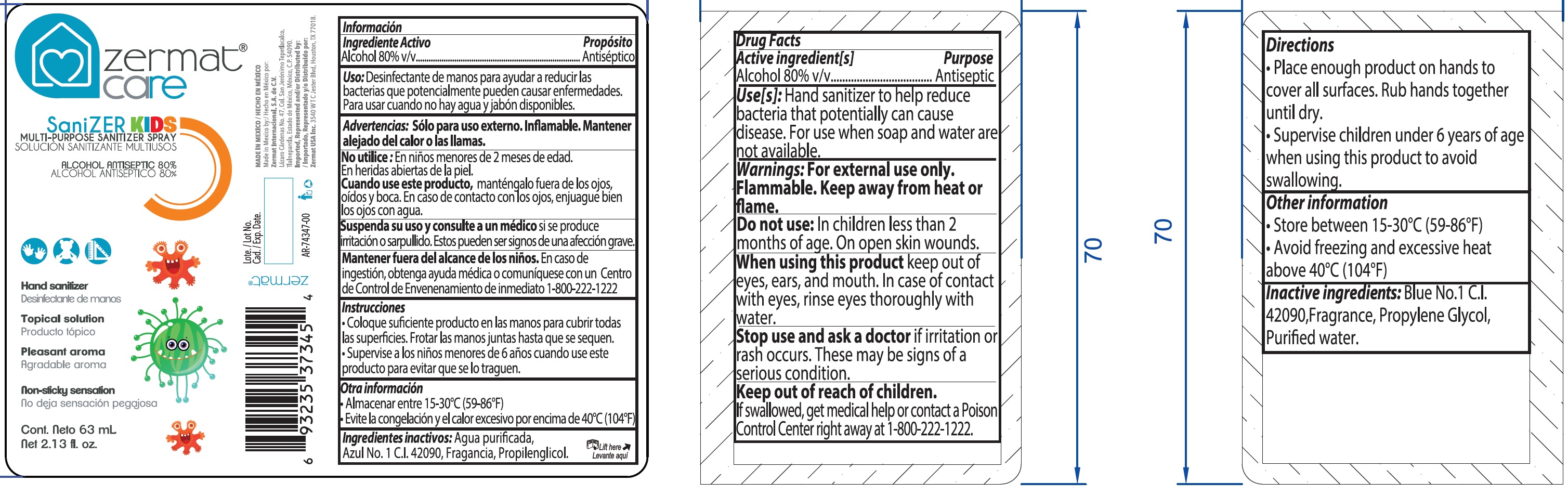
SANIZER KIDS Multi-Purpose Sanitizer Spray
SANIZER KIDS Multi-Purpose Sanitizer by
Drug Labeling and Warnings
SANIZER KIDS Multi-Purpose Sanitizer by is a Otc medication manufactured, distributed, or labeled by Zermat Internacional S.A. de C.V.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SANIZER KIDS MULTI-PURPOSE SANITIZER- alcohol spray
Zermat Internacional S.A. de C.V.
----------
SANIZER KIDS Multi-Purpose Sanitizer Spray
Use[s]:
Hand sanitizer to help reduce bacteria that potentially can cause disease. For use when soap and water are not available.
Warnings:
For external use only. Flammable. Keep away from heat or flame.
Directions
- Place enough product on hands to cover all surfaces. Rub hands together until dry.
- Supervise children under 6 years of age when using this product to avoid swallowing.
| SANIZER KIDS MULTI-PURPOSE SANITIZER
alcohol spray |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Zermat Internacional S.A. de C.V. (812820712) |
Revised: 6/2024
Document Id: 1a12b3b6-538a-05b9-e063-6394a90a60bf
Set id: 43c356bd-4a2f-4dc3-9615-b0a04430d57c
Version: 4
Effective Time: 20240604