VANAPAIN- choline salicylate, caffeine liquid
Vanapain by
Drug Labeling and Warnings
Vanapain by is a Otc medication manufactured, distributed, or labeled by GM Pharmaceuticals, Inc., Woodfield Pharmaceutical, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Salicylates (NSAIDs) may cause a severe allergic reaction which may include:
- hives
- skin reddening
- rash
- facial swelling
- shock
- asthma (wheezing)
Stomach bleeding warning:
This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Do not use if you ever had an allergic reaction to salicylates (including aspirin) or any other pain reliever/ fever reducer.
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- you are taking a diuretic
- you have a history of stomach problems such as heartburn, upset stomach, stomach pain, or ulcers
- you have asthma
Ask a doctor or pharmacist before use if
- under a doctor's care for any serious condition
- taking a prescription drug for diabetes, gout, or arthritis
When using this product
- limit the use of caffeine containing medications, foods, or beverages because too much caffeine may cause nervousness, irritability, sleeplessness, and occasionally, rapid heart beat
- the recommended dose of this product contains about as much caffeine as a cup of coffee
- take with food or milk if stomach upset occurs
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- symptoms do not improve
- pain gets worse or lasts more than 10 days
- ringing in the ears or loss of hearing occurs
- redness or swelling is present
- any new symptoms appear
- fever gets worse or lasts more than 3 days
- Directions
- Other Information
- Inactive Ingredients
- Questions? Comments?
- SPL UNCLASSIFIED SECTION
-
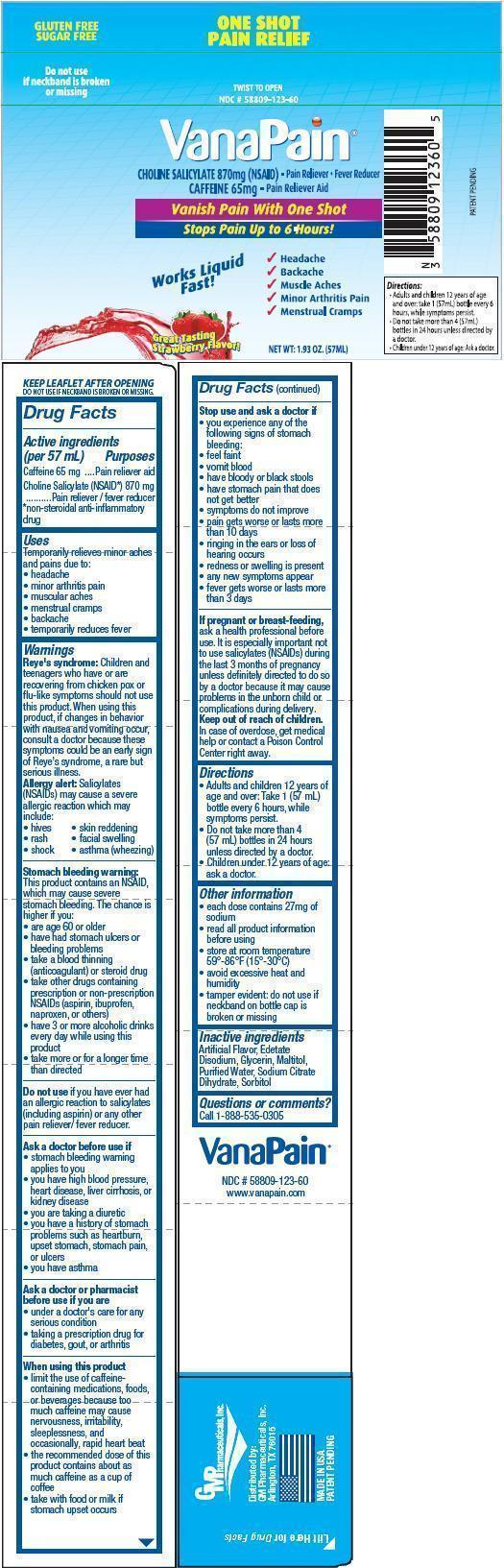
PRINCIPAL DISPLAY PANEL - 57mL Bottle Label
ONE SHOT PAIN RELIEF
GLUTEN FREE
SUGAR FREE
TWIST TO OPEN
Do not use if neckband is broken or missing
NDC # 58809-123-60
VanaPain ®
CHOLINE SALICYLATE 870mg (NSAID) - Pain Reliver / Fever Reducer
CAFFEINE 65mg - Pain Reliever Aid
Vanish Pain With One Shot
Stops Pain Up to 6 Hours
- Headache
- Backache
- Muscle Aches
- Minor Arthritis Pain
- Menstrual Cramps
Works Liquid Fast!
Great Tasting Strawberry Flavor!
NET WT: 1.93 OZ. (57ML)
Directions:
- Adults and children 12 years of age and over: take 1 (57mL) bottle every 6 hours, while symptoms persist.
- Do not take more than 4 (57mL) bottles in 24 hours unless directed by a doctor.
- Children under 12 years of age: Ask a doctor.
-
INGREDIENTS AND APPEARANCE
VANAPAIN
choline salicylate, caffeine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 58809-123 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHOLINE SALICYLATE (UNII: KD510K1IQW) (SALICYLIC ACID - UNII:O414PZ4LPZ) CHOLINE SALICYLATE 870 mg in 57 mL CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 65 mg in 57 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) MALTITOL (UNII: D65DG142WK) WATER (UNII: 059QF0KO0R) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SORBITOL (UNII: 506T60A25R) Product Characteristics Color Score Shape Size Flavor STRAWBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58809-123-60 57 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part343 07/22/2013 Labeler - GM Pharmaceuticals, Inc. (793000860) Establishment Name Address ID/FEI Business Operations Woodfield Pharmaceutical, LLC 079398730 manufacture(58809-123)
Trademark Results [Vanapain]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VANAPAIN 85739331 4489250 Live/Registered |
GM Pharmaceuticals, Inc. 2012-09-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.