CAVOREAL PLUS SALICYLIC ACID ANTI ACNE WITH BHA HA- salicylic acid cream
CAVOREAL Plus Salicylic Acid Anti Acne with BHA HA by
Drug Labeling and Warnings
CAVOREAL Plus Salicylic Acid Anti Acne with BHA HA by is a Otc medication manufactured, distributed, or labeled by Guangzhou Meijiao Medical Biotechnology Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
INGREDIENTS
Purified Water, Glycerin,Sodium Hyaluronate (Low Molecular Weight),Butylene Glycol (Plant-Derived), AcetylateSodium Hyaluronate, Aloe Vera Extract,Squalane (Plant-Derived), Jojoba Oil,Sunflower Seed Oil, Candelilla Wax,Titanium Dioxide (Coated), Tinosorb S,Uvinul A Plus, Uvinul T150, Octocrylene,Polyglyceryl-6 Distearate , Betaine,Alpha-Arbutin, Tocopherol (Vitamin E),Acetyl Tetrapeptide-15, Green Tea Extract,Rosehip Oil - PURPOSE
- WARNINGS
- DO NOT USE
- KEEP OUT OF REACH OF CHILDREN
- STOP USE
- INDICATIONS & USAGE
- WHEN USING
- DOSAGE & ADMINISTRATION
-
PRINCIPAL DISPLAY PANEL
 Use: Apply to your clean faee $o minutesbelore sun exposure, can bse used daily
Use: Apply to your clean faee $o minutesbelore sun exposure, can bse used daily
SANDRA PLASENCIA
GREEN TEADaily SPF 5O
MOISTURIZER
Alpha Arbutin- Aloe Wern-SqualaneHIGH PROTECTION
UVA UVB PA++++
FERMENTED
SHINCARE
VIONISVId VHGNVS
INGREDIENTS :
Purified water, lycerin.Sodium Hvaluronate (Low Mlolecular weight).Butylemne Glyeol (Plant-Derived), AcetylaceSodium Hylummate, Aloe Vera Exerace,Squalane(Plant crived) JojobCl.Sunikwer Seed 0il, ('andelilla wax,Titanium Dioxide (oatel], inosorb S,UvinulA Pus,UviuTT150,OccocryinePolyglyeeryl-6 Distearate . Betaine,Alpls-Arbuun, Tocopberol (Vitamin E).Acetyl"letrpeptide.16,(iren 'ea Extract,Raschip Cl
CAUTION:
For external use only Avoid directcotact with eves,If irritatmm occurs, stopuse and consult a doctor if msled.putch test recommicnded. J)o not useif wi are alkrgic to any ofthe ingredients.kecp out of reach of children.
Mamunctured in PRc f casa SandrnChicage,[l.60625
0778-854-590$
www.sandraplasencia.com -
INGREDIENTS AND APPEARANCE
CAVOREAL PLUS SALICYLIC ACID ANTI ACNE WITH BHA HA
salicylic acid creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 87036-032 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 1 g in 15 mL Inactive Ingredients Ingredient Name Strength ASTRAGALUS MEMBRANACEUS ROOT POWDER (UNII: 922OP8YUPF) SORBITAN OLEATE (UNII: 06XEA2VD56) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) TAPIOCA STARCH (UNII: 24SC3U704I) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) CENTELLA ASIATICA (UNII: 7M867G6T1U) WATER O-15 (UNII: 63M8RYN44N) TRITICUM VULGARE (WHEAT) GERM (UNII: YR3G369F5A) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALFUZOSIN HYDROXY ACID (UNII: LHE12Y00R8) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) CETEARYL ALCOHOL (UNII: 2DMT128M1S) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) HELIANTHUS ANNUUS (SUNFLOWER) SEED OIL (UNII: 3W1JG795YI) ISOHEXADECANE (UNII: 918X1OUF1E) GLYCERYL STEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) GLYCERIN (UNII: PDC6A3C0OX) POLYSORBATE 80 (UNII: 6OZP39ZG8H) HYALURONIC ACID (UNII: S270N0TRQY) PANTHENOL (UNII: WV9CM0O67Z) BISABOLOL (UNII: 24WE03BX2T) TREHALOSE (UNII: B8WCK70T7I) OPUNTIA FICUS-INDICA (UNII: 23Z87HTQ6P) NIACINAMIDE (UNII: 25X51I8RD4) SAPOSHNIKOVIA DIVARICATA ROOT (UNII: 8H84LFK2QD) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ALBIZIA JULIBRISSIN FLOWER (UNII: 071O9775L9) GASTRODIA ELATA TUBER (UNII: 08F85I5YAV) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) PENTYLENE GLYCOL (UNII: 50C1307PZG) ROSMARINUS OFFICINALIS (ROSEMARY) LEAF OIL (UNII: 8LGU7VM393) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 87036-032-01 15 mL in 1 TUBE; Type 0: Not a Combination Product 11/18/2025 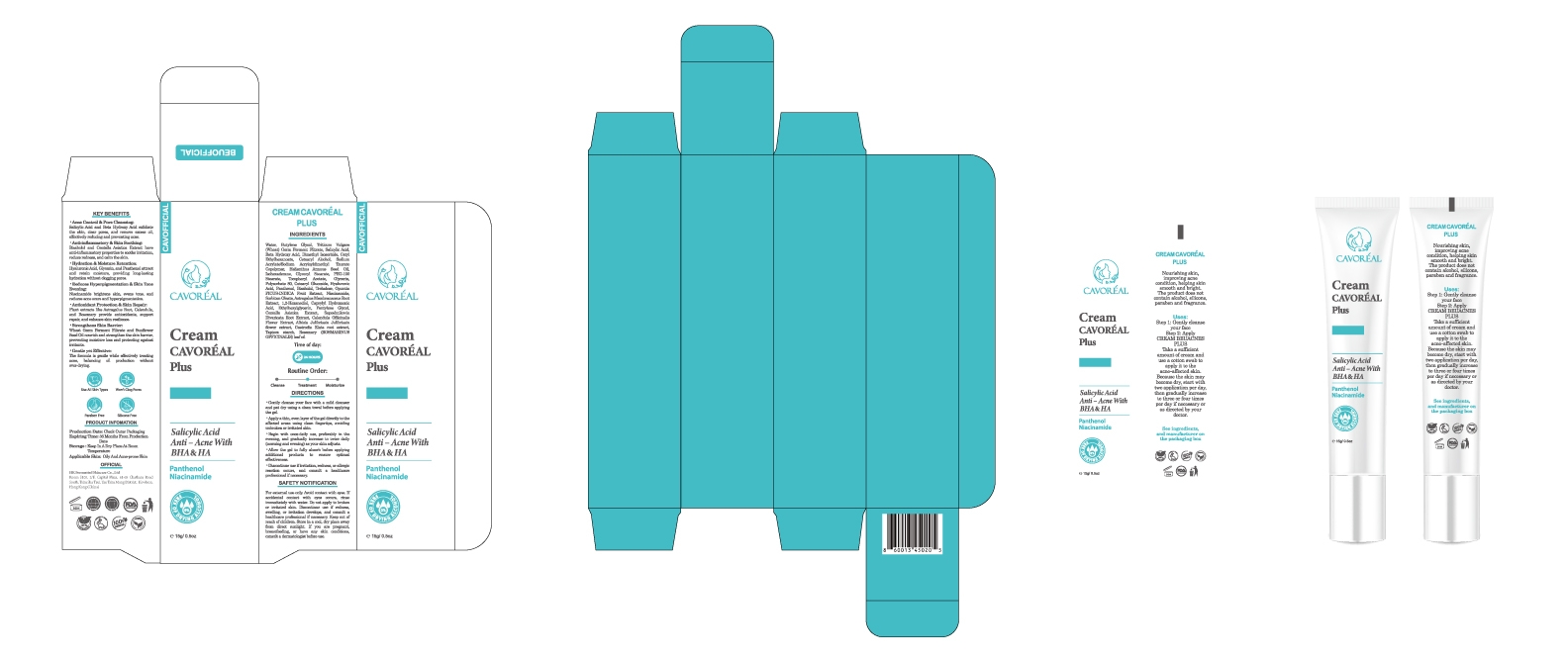
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 08/17/2025 Labeler - Guangzhou Meijiao Medical Biotechnology Co., Ltd. (978211825) Establishment Name Address ID/FEI Business Operations Guangzhou Meijiao Medical Biotechnology Co., Ltd. 978211825 manufacture(87036-032)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.