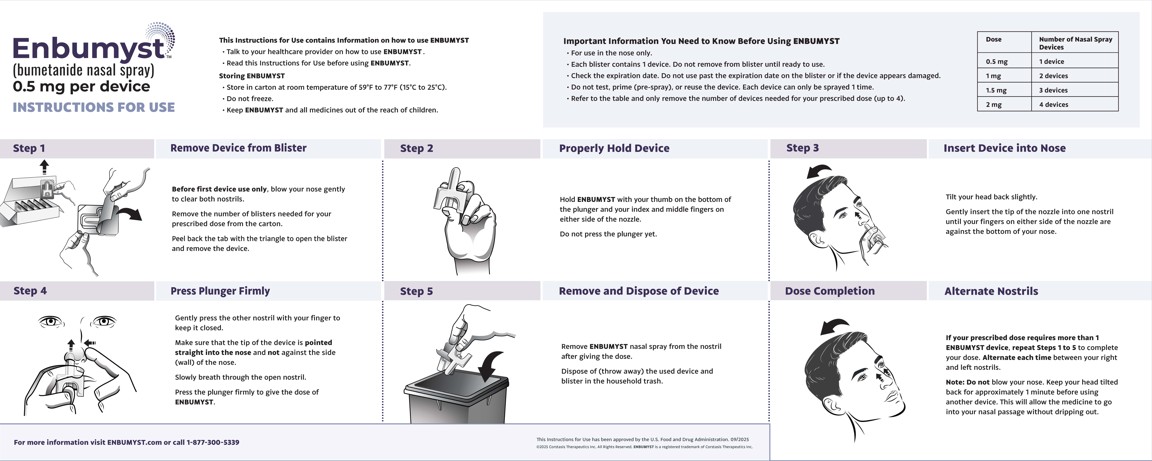
Enbumyst (Bumetanide Nasal Spray)
Enbumyst by
Drug Labeling and Warnings
Enbumyst by is a Prescription medication manufactured, distributed, or labeled by Renaissance Lakewood LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ENBUMYST- bumetanide nasal spray spray
Renaissance Lakewood LLC
----------
Enbumyst (Bumetanide Nasal Spray)
| ENBUMYST
bumetanide nasal spray spray |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Renaissance Lakewood LLC (077744035) |
Revised: 1/2026
Document Id: 48734676-fadd-ee0b-e063-6294a90a5d68
Set id: 4409c4a5-2090-7cb3-e063-6394a90a0872
Version: 3
Effective Time: 20260115