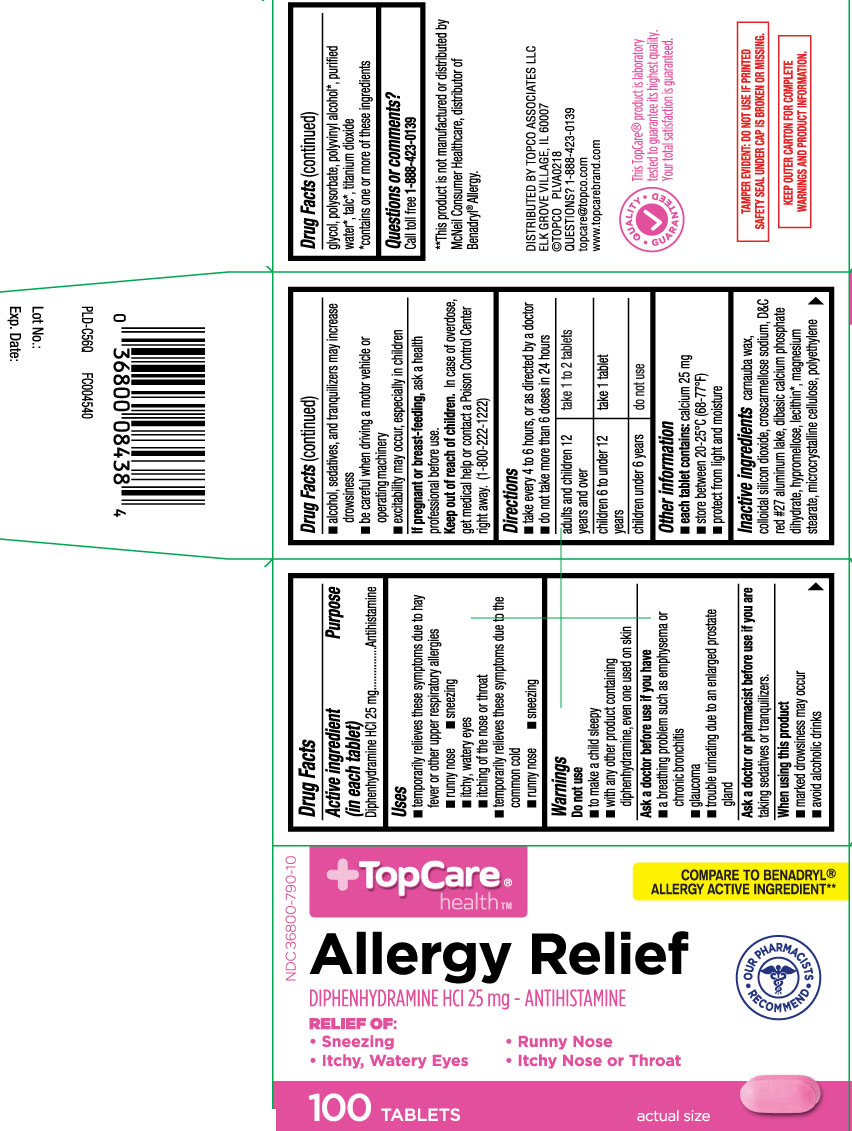
ALLERGY RELIEF- diphenhydramine hcl tablet
Allergy Relief by
Drug Labeling and Warnings
Allergy Relief by is a Otc medication manufactured, distributed, or labeled by TOP CARE (Topco Associates LLC). Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
-
a breathing problem such as emphysema or chronic bronchitis
-
glaucoma
-
trouble urinating due to an enlarged prostate gland
- Directions
- Other information
-
Inactive ingredients
carnauba wax, colloidal silicon dioxide, croscarmellose sodium, D&C Red #27 Aluminum Lake, dibasic calcium phosphate dihydrate, hypromellose, lecithin*, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate, polyvinyl alcohol*, *purified water, *talc, and titanium dioxide
* Contains one or more of these ingredients
- Questions or comments?
-
Principal Display Panel
COMPARE TO BENADRYL® ALLERGY ACTIVE INGREDIENT**
Allergy Relief
DIPHENHYDRAMINE HCI 25 mg - ANTIHISTAMINE
RELIEF OF:
- Sneezing
- Runny Nose
- Itchy, Watery Eyes
- Itchy Nose or Throat
TABLETS
*This product is not manufactured or distributed by McNeil Consumer Healthcare, distributor of Benadryl® Allergy.
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
DISTRIBUTED BY TOPCO ASSOCIATED LLC
ELK GROVE VILLAGE, IL 60007
©TOPCO PLVA0218
- Product Label
-
INGREDIENTS AND APPEARANCE
ALLERGY RELIEF
diphenhydramine hcl tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 36800-790 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) D&C RED NO. 27 (UNII: 2LRS185U6K) CALCIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: O7TSZ97GEP) HYPROMELLOSES (UNII: 3NXW29V3WO) EGG PHOSPHOLIPIDS (UNII: 1Z74184RGV) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) WATER (UNII: 059QF0KO0R) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color PINK Score no score Shape CAPSULE Size 11mm Flavor Imprint Code T;061;V;25;S4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 36800-790-10 1 in 1 BOX 02/28/2018 1 100 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 36800-790-40 400 in 1 BOTTLE; Type 0: Not a Combination Product 02/28/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 02/28/2018 Labeler - TOP CARE (Topco Associates LLC) (006935977)
Trademark Results [Allergy Relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALLERGY RELIEF 98236984 not registered Live/Pending |
Dmytro Kononenko 2023-10-24 |
 ALLERGY RELIEF 90457167 not registered Live/Pending |
American Textile Company, Inc. 2021-01-10 |
 ALLERGY RELIEF 78838437 3358249 Live/Registered |
Meshbesher Health Corporation 2006-03-16 |
 ALLERGY RELIEF 76619855 3066888 Live/Registered |
AMERICAN TEXTILE COMPANY 2004-11-09 |
 ALLERGY RELIEF 74668018 not registered Dead/Abandoned |
NaturaLife Corporation 1995-05-01 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.