® Tablets
Lubricant PM by
Drug Labeling and Warnings
Lubricant PM by is a Otc medication manufactured, distributed, or labeled by Bryant Ranch Prepack. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
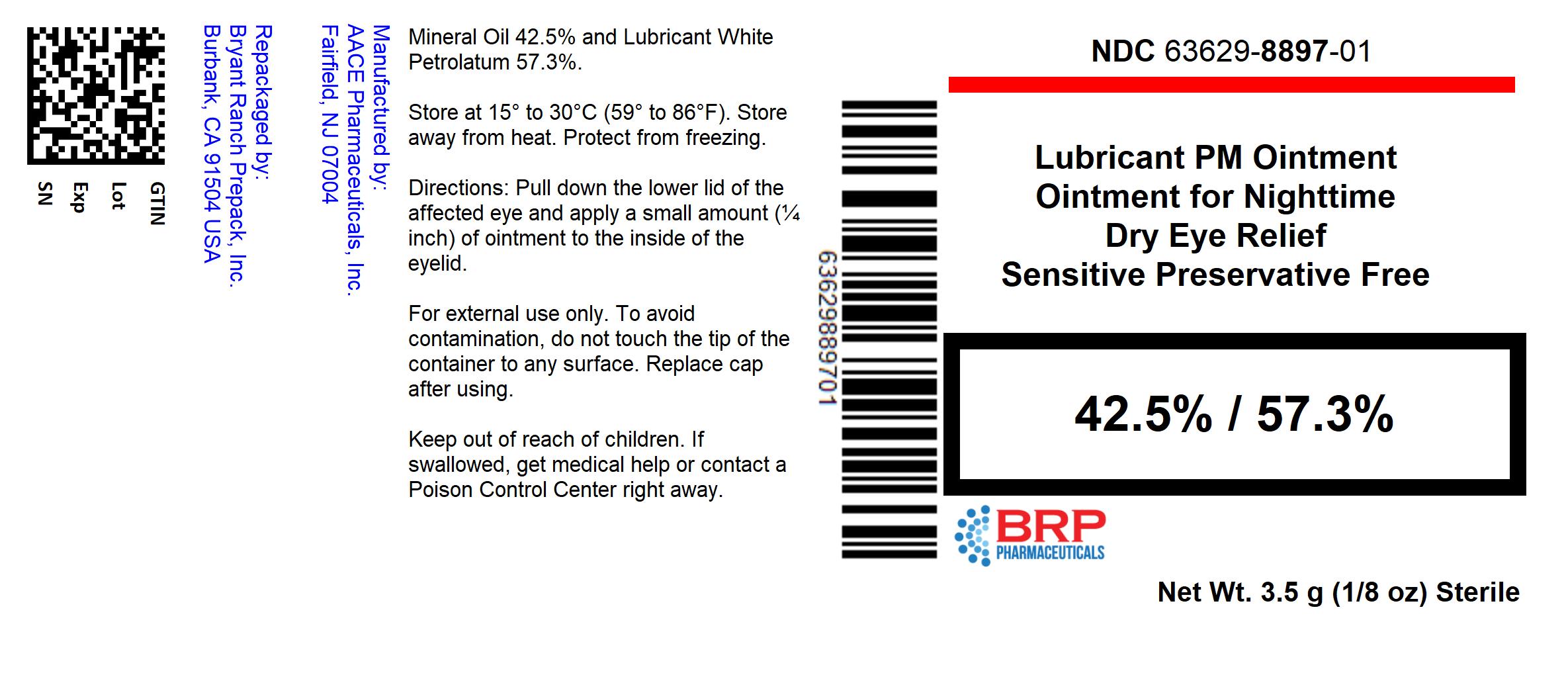
LUBRICANT PM- mineral oil, white petrolatum lubricant eye ointment ointment
Bryant Ranch Prepack
----------
®Tablets
SPL UNCLASSIFIED SECTION
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
USES
- For the temporary relief of burning, irritation and discomfort due to dryness of the eye or exposure to wind and sun.
- May be used as a protectant against further irritation.
WARNINGS
- For external use only.
- To avoid contamination, do not touch the tip of container to any surface. Replace cap after using.
Stop use and ask a doctor if
- you feel eye pain.
- changes in vision occur.
- redness or irritation of the eye(s) gets worse, persists or lasts more than 72 hours.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
- Pull down the lower lid of the affected eye and apply a small amount (one fourth inch) of ointment to the inside of the eyelid.
OTHER INFORMATION
- Store away from heat.
- Protect from freezing.
- Use only if tape seals on top and bottom flaps are intact.
- Use before expiration date marked on container.
- Store at 15° to 30°C (59° to 86°F).
RETAIN THIS CARTON FOR FUTURE REFERENCE.
HOW SUPPLIED
Lubricant PM Eye Ointment
- NDC: 63629-8897-1: 3.5 g in a TUBE
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504
| LUBRICANT PM
mineral oil, white petrolatum lubricant eye ointment ointment |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Bryant Ranch Prepack (171714327) |
| Registrant - Bryant Ranch Prepack (171714327) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Bryant Ranch Prepack | 171714327 | REPACK(63629-8897) , RELABEL(63629-8897) | |
Revised: 9/2024
Document Id: d59afa2b-2d64-4e53-a0f0-18e53b93e04b
Set id: 445f4089-53af-4f75-9612-8664a0a221cd
Version: 105
Effective Time: 20240925
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.