PROPARACAINE HYDROCHLORIDE- proparacaine hydrochloride solution/ drops
Proparacaine Hydrochloride by
Drug Labeling and Warnings
Proparacaine Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Sandoz Inc., Alcon Laboratories, Inc., Alcon Research LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Proparacaine hydrochloride ophthalmic solution 0.5% is a topical local anesthetic for ophthalmic use. The active ingredient is represented by the structural formula:
Established name: Proparacaine Hydrochloride
Chemical name: Benzoic acid, 3-amino-4-propoxy-,2-(diethylamino) ethyl ester, monohydrochloride.
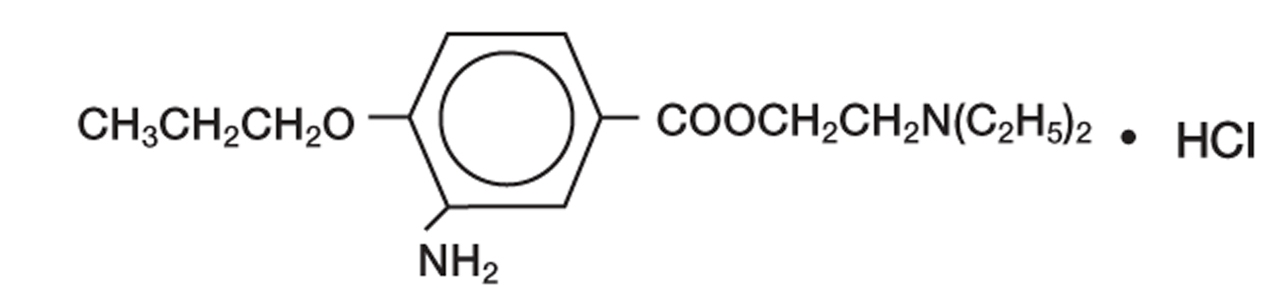
Molecular Weight: 330.85
Each mL contains: Active: proparacaine hydrochloride 5mg 0.5%. Preservative: benzalkonium chloride (0.01%). Inactives: glycerin; and purified water. The pH may be adjusted with hydrochloric acid and/or sodium hydroxide.
- CLINICAL PHARMACOLOGY
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate carcinogenic potential, mutagenicity, or possible impairment of fertility in males or females.
Pregnancy
Pregnancy Category C: Animal reproduction studies have not been conducted with proparacaine hydrochloride ophthalmic solution 0.5%. It is also not known whether proparacaine hydrochloride can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Proparacaine hydrochloride should be administered to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when proparacaine hydrochloride is administered to a nursing woman.
Pediatric Use
Safety and effectiveness of proparacaine hydrochloride ophthalmic solution in pediatric patients have been established. Use of proparacaine hydrochloride is supported by evidence from adequate and well-controlled studies in adults and children over the age of twelve, and safety information in neonates and other pediatric patients.
-
ADVERSE REACTIONS
Occasional temporary stinging, burning and conjunctival redness may occur with the use of proparacaine. A rare, severe, immediate-type, apparently hyperallergic corneal reaction characterized by acute, intense and diffuse epithelial keratitis, a gray, ground glass appearance, sloughing of large areas of necrotic epithelium, corneal filaments and, sometimes, iritis with descemetitis has been reported.
Allergic contact dermatitis from proparacaine with drying and fissuring of the fingertips has also been reported.
-
DOSAGE AND ADMINISTRATION
Usual Dosage: Removal of foreign bodies and sutures, and for tonometry: 1 to 2 drops (in single instillations) in each eye before operating.
Short corneal and conjunctival procedures: 1 drop in each eye every 5 to 10 minutes for 5 to 7 doses.
NOTE: Proparacaine hydrochloride ophthalmic solution should be clear to straw-color. If the solution becomes darker, discard the solution.
- HOW SUPPLIED
-
SPL UNCLASSIFIED SECTION
To report SUSPECTED ADVERSE REACTIONS, contact Sandoz Inc., at 1-800-525-8747 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
*DROP-TAINER is a registered trademark of Alcon Research, Ltd.
Rev. June 2012
SANDOZ
Manufactured by
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 for
Sandoz Inc.
Princeton, NJ 08540
Printed in USA9007253-1011
-
PRINCIPAL DISPLAY PANEL
NDC 61314-016-01
Proparacaine Hydrochloride Ophthalmic Solution, USP
0.5%
Rx only
STERILE
15 mL
PRECAUTION: NOT FOR INJECTION. FOR TOPICAL OPHTHALMIC USE ONLY. Do not touch dropper tip to any surface, as this may contaminate the solution.
USUAL DOSAGE: 1 or 2 drops.
Read enclosed insert.
Bottles must be stored in unit
carton to protect from light.
STORAGE: Store between 2°-8°C (36°-46°F). Proparacaine hydrochloride ophthalmic solution should be clear to straw-color. If the solution becomes darker, discard the solution.
INGREDIENTS: Each mL contains:
Active: proparacaine hydrochloride 5 mg (0.5%).
Preservative: benzalkonium chloride 0.01%.
Inactives: glycerin, hydrochloric acid and/or sodium hydroxide (to adjust pH), purified water.
MANUFACTURED BY ALCON LABORATORIES, INC.
Manufactured by
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 for
Sandoz Inc.
Princeton, NJ 08540
Printed in USA
9012981-0216
Rev. 06/2012
NDC 61314-016-01
Proparacaine Hydrochloride Ophthalmic Solution, USP
0.5%
Rx only
STERILE
15 mL
SANDOZ
INGREDIENTS: Each mL contains:
Active: proparacaine hydrochloride 5 mg (0.5%).
PRECAUTION: NOT FOR INJECTION. FOR TOPICAL OPHTHALMIC USE ONLY. Do not touch dropper tip to any surface as this may contaminate the solution.
USUAL DOSAGE: 1 or 2 drops.
Read enclosed insert.
STORAGE: Store between 2º-8ºC (36º-46ºF).
Bottles must be stored in unit carton to protect from light.
Proparacaine hydrochloride ophthalmic solution should be clear to straw-color. If the solution becomes darker, discard the solution.
Manufactured by
Alcon Laboratories, Inc.
Fort Worth, Texas 76134 for
Sandoz Inc.
Princeton, NJ 08540
Rev. 04-2012
LOT/EXP.:
H14233-0216

-
INGREDIENTS AND APPEARANCE
PROPARACAINE HYDROCHLORIDE
proparacaine hydrochloride solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 61314-016 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PROPARACAINE HYDROCHLORIDE (UNII: U96OL57GOY) (PROPARACAINE - UNII:B4OB0JHI1X) PROPARACAINE HYDROCHLORIDE 5 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 61314-016-01 1 in 1 CARTON 06/05/2000 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA080027 06/05/2000 Labeler - Sandoz Inc. (005387188) Registrant - Alcon Laboratories, Inc. (008018525) Establishment Name Address ID/FEI Business Operations Alcon Research LLC 007672236 manufacture(61314-016)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.