LIVALO- pitavastatin calcium tablet, film coated
Livalo by
Drug Labeling and Warnings
Livalo by is a Prescription medication manufactured, distributed, or labeled by Kowa Pharmaceuticals America, Inc., Kowa Corporation Ltd., Patheon Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use LIVALO® safely and effectively. See full prescribing information for LIVALO.
LIVALO (pitavastatin) tablets, for oral use
Initial U.S. Approval: 2009INDICATIONS AND USAGE
LIVALO is a HMG-CoA reductase inhibitor indicated as an adjunctive therapy to diet in:
- Adult patients with primary hyperlipidemia or mixed dyslipidemia to reduce elevated total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), apolipoprotein B (Apo B), triglycerides (TG), and to increase high-density lipoprotein cholesterol (HDL-C) (1).
- Pediatric patients aged 8 years and older with heterozygous familial hypercholesterolemia (HeFH) to reduce elevated TC, LDL-C, and Apo B (1).
Limitations of Use:
- The effect of LIVALO on cardiovascular morbidity and mortality has not been determined.
DOSAGE AND ADMINISTRATION
- Take LIVALO orally once daily with or without food at the same time each day (2.1).
- Individualize the dose of LIVALO according to patient characteristics, goal of therapy, and response (2.1).
- After initiation or upon titration, analyze lipid levels after 4 weeks and adjust the dosage accordingly (2.1).
- The recommended starting LIVALO dosage is 2 mg once daily (2.2).
- The maximum recommended dosage is LIVALO 4 mg once daily (2.2).
- The recommended staring dosage for adults with moderate and severe renal impairment (estimated glomerular filtration rate 30-59 and 15-29 mL/min/1.73 m2, respectively) as well as end-stage renal disease on hemodialysis is LIVALO 1 mg once daily and the maximum dose is 2 mg once daily (2.3)
- There are LIVALO dosage adjustments due to drug interactions for:
DOSAGE FORMS AND STRENGTHS
- Tablets: 1 mg, 2 mg, and 4 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Myopathy and Rhabdomyolysis: Risk factors include age 65 and greater, renal impairment, inadequately treated hypothyroidism, and higher doses of LIVALO. Discontinue LIVALO if markedly elevated CK levels occur or myopathy is diagnosed or suspected. Temporarily discontinue LIVALO in patients experiencing an acute or serious condition at high risk of developing renal failure secondary to rhabdomyolysis, e.g., sepsis; shock; severe hypovolemia; major surgery; trauma; severe metabolic, endocrine, or electrolyte disorders; or uncontrolled epilepsy. Inform patients of the risk of myopathy and rhabdomyolysis when starting or increasing the LIVALO dosage. Instruct patients to promptly report any unexplained muscle pain, tenderness or weakness particularly if accompanied by malaise or fever (5.1).
- Immune-Mediated Necrotizing Myopathy (IMNM): There have been rare reports of IMNM, an autoimmune myopathy, associated with statin use. IMNM is characterized by: proximal muscle weakness and elevated serum creatine kinase, which persist despite discontinuation of statin treatment; muscle biopsy showing necrotizing myopathy without significant inflammation; and improvement with immunosuppressive agents (5.2).
- Hepatic Dysfunction: Increases in serum transaminases can occur. Rare postmarketing reports of fatal and non-fatal hepatic failure have occurred. Consider liver enzyme testing before initiating therapy and as clinically indicated thereafter. If serious hepatic injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs, promptly discontinue LIVALO (5.3)
- Increases in HbA1c and Fasting Serum Glucose Levels: Have been reported with statins, including LIVALO. Optimize lifestyle measures, including regular exercise, maintaining a healthy body weight, and making healthy food choices. (5.4)
ADVERSE REACTIONS
The most frequent adverse reactions (rate ≥ 2%) were myalgia, back pain, diarrhea, constipation and pain in extremity. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Kowa Pharmaceuticals America, Inc. at 1-877-334-3464 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Gemfibrozil: Avoid concomitant use with LIVALO (7)
- Fibrates: Consider if the benefit of using fibrates concomitantly with LIVALO outweighs the increased risk of myopathy and rhabdomyolysis (7)
- Niacin: Consider if the benefit of using lipid-modifying doses (>1 g/day) of niacin concomitantly with LIVALO outweighs the increased risk of myopathy and rhabdomyolysis (7)
- Colchicine: Consider the risk/benefit of concomitant use with LIVALO (7)
USE IN SPECIFIC POPULATIONS
- Females of Reproductive Potential: Advise females to use effective contraception during treatment. (8.3)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 General Dosage and Administration Information
2.2 Recommended Dosage for Adults and Pediatric Patients Aged 8 Years and Older
2.3 Recommended Dosage in Patients with Renal Impairment
2.4 LIVALO Dosage Adjustments Due to Drug Interactions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Myopathy and Rhabdomyolysis
5.2 Immune-Mediated Necrotizing Myopathy
5.3 Hepatic Dysfunction
5.4 Increases in HbA1c and Fasting Serum Glucose Levels
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Primary Hyperlipidemia or Mixed Dyslipidemia in Adult Patients
14.2 HeFH in Pediatric Patients
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
LIVALO is indicated as an adjunctive therapy to diet in:
- Adult patients with primary hyperlipidemia or mixed dyslipidemia to reduce elevated total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), apolipoprotein B (Apo B), triglycerides (TG), and to increase high-density lipoprotein cholesterol (HDL-C).
- Pediatric patients aged 8 years and older with heterozygous familial hypercholesterolemia (HeFH) to reduce elevated TC, LDL-C, and Apo B.
Limitations of Use
- The effect of LIVALO on cardiovascular morbidity and mortality has not been determined.
-
2 DOSAGE AND ADMINISTRATION
2.1 General Dosage and Administration Information
- Take LIVALO orally once daily with or without food at the same time each day.
- Individualize the dose of LIVALO according to patient characteristics, goal of therapy, and response.
- After initiation or upon titration of LIVALO, analyze lipid levels after 4 weeks and adjust the dosage accordingly.
2.2 Recommended Dosage for Adults and Pediatric Patients Aged 8 Years and Older
- The recommended starting LIVALO dosage is 2 mg once daily.
- The maximum recommended dosage is LIVALO 4 mg once daily [see Warnings and Precautions (5.1)].
2.3 Recommended Dosage in Patients with Renal Impairment
- The recommended starting dose for adults with moderate and severe renal impairment (estimated glomerular filtration rate 30 – 59 mL/minute/1.73 m2 and 15 – 29 mL/minute/1.73 m2, respectively) and patients with end-stage renal disease receiving hemodialysis is LIVALO 1 mg once daily.
- The maximum recommended dose for these patients is LIVALO 2 mg once daily [see Use in Specific Populations (8.6)].
- A recommended dosage for pediatric patients with renal impairment has not been established.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
LIVALO is contraindicated in the following conditions:
- Known hypersensitivity to pitavastatin or any inactive ingredient in LIVALO. Hypersensitivity reactions including rash, pruritus, and urticaria have been reported with LIVALO [see Adverse Reactions (6.1)].
- Concomitant use of cyclosporine [see Drug Interactions (7)].
- Active liver disease including unexplained persistent elevations of hepatic transaminase levels [see Warnings and Precautions (5.3)].
- Pregnancy [see Use in Specific Populations (8.1, 8.3)].
- Lactation. It is not known if pitavastatin is present in human milk; however, another drug in this class passes into breast milk. Since HMG-CoA reductase inhibitors have the potential for serious adverse reactions in breastfed infants, females who require pitavastatin treatment should not breastfeed their infants [see Use in Specific Populations (8.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Myopathy and Rhabdomyolysis
LIVALO may cause myopathy (muscle pain, tenderness, or weakness with creatine kinase (CK) above ten times the upper limit of normal) and rhabdomyolysis (with or without acute renal failure secondary to myoglobinuria). Rare fatalities have occurred as a result of rhabdomyolysis with statin use, including LIVALO.
Risk Factors for Myopathy
Risk factors for myopathy include age 65 years or greater, uncontrolled hypothyroidism, renal impairment, concomitant use of certain drugs, and higher LIVALO dosage. Dosages of LIVALO greater than 4 mg once daily were associated with an increased risk for severe myopathy in premarketing clinical studies. The maximum recommended dose of LIVALO is 4 mg once daily [see Dosage and Administration (2.2)].
Steps to Prevent or Reduce the Risk of Myopathy and Rhabdomyolysis
LIVALO is contraindicated in patients taking cyclosporine and not recommended in patients taking gemfibrozil [see Contraindications (4) and Drug Interactions (7)]. There are LIVALO dosage restrictions for patients taking erythromycin or rifampin [see Dosage and Administration (2.4)]. The following drugs when used concomitantly with LIVALO may also increase the risk of myopathy and rhabdomyolysis: lipid-modifying dosages of niacin (>1grams/day), fibrates, and colchicine [see Drug Interactions (7)].
Discontinue LIVALO if markedly elevated CK levels occur or myopathy is diagnosed or suspected. Muscle symptoms and CK increases may resolve if LIVALO is discontinued. Temporarily discontinue LIVALO in patients experiencing an acute or serious condition at high risk of developing renal failure secondary to rhabdomyolysis, e.g., sepsis; shock; severe hypovolemia; major surgery; trauma; severe metabolic, endocrine, or electrolyte disorders; or uncontrolled epilepsy.
Inform patients of the risk of myopathy and rhabdomyolysis when starting or increasing the LIVALO dosage. Instruct patients to promptly report any unexplained muscle pain, tenderness or weakness particularly if accompanied by malaise or fever.
5.2 Immune-Mediated Necrotizing Myopathy
There have been rare reports of immune-mediated necrotizing myopathy (IMNM), an autoimmune myopathy, associated with statin use. IMNM is characterized by: proximal muscle weakness and elevated serum creatine kinase, which persist despite discontinuation of statin treatment; muscle biopsy showing necrotizing myopathy without significant inflammation; and improvement with immunosuppressive agents. Additional neuromuscular and serologic testing may be necessary. Treatment with immunosuppressive agents may be required.
5.3 Hepatic Dysfunction
Increases in serum transaminases have been reported with LIVALO [see Adverse Reactions (6)]. In most cases, the elevations were transient and either resolved or improved on continued therapy or after a brief interruption in therapy. There have been rare postmarketing reports of fatal and non-fatal hepatic failure in patients taking statins, including LIVALO.
Patients who consume substantial quantities of alcohol and/or have a history of liver disease may be at increased risk for hepatic injury.
Consider liver enzyme testing before the initiation of LIVALO and thereafter, when clinically indicated. LIVALO is contraindicated in patients with active liver disease including unexplained persistent elevations in hepatic transaminase levels [see Contraindications (4)]. If serious hepatic injury with clinical symptoms and/or hyperbilirubinemia or jaundice occurs, promptly discontinue LIVALO.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in other sections of the labeling:
- Myopathy and Rhabdomyolysis [see Warnings and Precautions (5.1)]
- Immune-Mediated Necrotizing Myopathy [see Warning and Precautions (5.2)]
- Hepatic Dysfunction [see Warning and Precautions (5.3)]
- Increases in HbA1c and Fasting Serum Glucose Levels [see Warning and Precautions (5.4)].
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of one drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Adverse Reactions in Adults with Primary Hyperlipidemia and Mixed Dyslipidemia
In 10 controlled clinical studies and 4 subsequent open-label extension studies, 3,291 adult patients with primary hyperlipidemia or mixed dyslipidemia were administered LIVALO 1 mg to 4 mg daily. The mean continuous exposure of pitavastatin (1 mg to 4 mg) was 36.7 weeks (median 51.1 weeks). The mean age of the patients was 60.9 years (range; 18 years – 89 years) and the gender distribution was 48% males and 52% females. Approximately 93% of the patients were Caucasian, 7% were Asian/Indian, 0.2% were African American and 0.3% were Hispanic and other.
In controlled clinical studies and their open-label extensions, 3.9% (1 mg), 3.3% (2 mg), and 3.7% (4 mg) of LIVALO-treated patients were discontinued due to adverse reactions. The most common adverse reactions that led to treatment discontinuation were: elevated creatine phosphokinase (0.6% on 4 mg) and myalgia (0.5% on 4 mg).
Adverse reactions reported in ≥ 2% of patients in controlled clinical studies and at a rate greater than or equal to placebo are shown in Table 1. These studies had treatment duration of up to 12 weeks.
Table 1. Adverse Reactions ( ≥ 2% and ≥ placebo) in Adult Patients with Primary Hyperlipidemia and Mixed Dyslipidemia in Studies up to 12 Weeks Adverse
ReactionsPlacebo
(n= 208)
%LIVALO 1 mg
(n=309)
%LIVALO 2 mg
(n=951)
%LIVALO 4 mg
(n=1540)
%Back Pain 2.9 3.9 1.8 1.4 Constipation 1.9 3.6 1.5 2.2 Diarrhea 1.9 2.6 1.5 1.9 Myalgia 1.4 1.9 2.8 3.1 Pain in extremity 1.9 2.3 0.6 0.9 Other adverse reactions reported from clinical studies were arthralgia, headache, influenza, and nasopharyngitis.
Hypersensitivity reactions including rash, pruritus, and urticaria have been reported with LIVALO.
The following laboratory abnormalities have been reported: elevated creatine phosphokinase, transaminases, alkaline phosphatase, bilirubin, and glucose.
Adverse Reactions in Adult HIV-Infected Patients with Dyslipidemia
In a double-blind, randomized, controlled, 52-week trial, 252 HIV-infected patients with dyslipidemia were treated with either LIVALO 4 mg once daily (n=126) or another statin (n=126). All patients were taking antiretroviral therapy (excluding darunavir) and had HIV-1 RNA less than 200 copies/mL and CD4 count greater than 200 cell/μL for at least 3 months prior to randomization. The safety profile of LIVALO was generally consistent with that observed in the clinical trials described above. One patient (0.8%) treated with LIVALO had a peak creatine phosphokinase value exceeding 10 times the upper limit of normal (ULN), which resolved spontaneously. Four patients (3%) treated with LIVALO had at least one ALT value exceeding 3 times but less than 5 times the ULN, none of which led to drug discontinuation. Virologic failure was reported for four patients (3%) treated with LIVALO, defined as a confirmed measurement of HIV-1 RNA exceeding 200 copies/mL that was also more than a 2-fold increase from baseline.
Adverse Reactions in Pediatric Patients Aged 8 Years and Older with HeFH
In a 12-week, double-blind, placebo-controlled trial of LIVALO 1 mg, 2 mg, and 4 mg once daily in 82 pediatric patients 8 years to 16 years of age with HeFH and a 52-week open-label trial in 85 pediatric patients with HeFH, the safety profile was similar to that observed in the adult population.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of LIVALO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Gastrointestinal disorders: abdominal discomfort, abdominal pain, dyspepsia, nausea
- General disorders: asthenia, fatigue, malaise, dizziness
- Hepatobiliary disorders: hepatitis, jaundice, fatal and non-fatal hepatic failure
- Immune system disorders: immune-mediated necrotizing myopathy associated with statin use
- Metabolism and nutrition disorders: increases in HbA1c, fasting serum glucose levels
- Musculoskeletal and connective tissue disorders: muscle spasms, myopathy, rhabdomyolysis
- Nervous system disorders: hypoesthesia, peripheral neuropathy
- Psychiatric disorders: insomnia, depression. Rare reports of cognitive impairment (e.g., memory loss, forgetfulness, amnesia, memory impairment, confusion) associated with statin use. Cognitive impairment was generally nonserious, and reversible upon statin discontinuation, with variable times to symptom onset (1 day to years) and symptom resolution (median of 3 weeks).
- Reproductive system and breast disorders: erectile dysfunction
- Respiratory, thoracic and mediastinal disorders: interstitial lung disease
-
7 DRUG INTERACTIONS
Drug Interactions that Increase the Risk of Myopathy and Rhabdomyolysis with LIVALO
Table 2 includes a list of drugs that increase the risk of myopathy and rhabdomyolysis when administered concomitantly with LIVALO and instructions for preventing or managing drug interactions [see Warnings and Precautions (5.1), Clinical Pharmacology (12.3)].
Table 2: Drug Interactions that Increase the Risk of Myopathy and Rhabdomyolysis with LIVALO Cyclosporine Clinical Impact: Cyclosporine significantly increases pitavastatin exposure and increases the risk of myopathy and rhabdomyolysis. Intervention: Concomitant use of cyclosporine with LIVALO is contraindicated [see Contraindications (4)]. Gemfibrozil Clinical Impact: Gemfibrozil may cause myopathy when given alone. The risk of myopathy and rhabdomyolysis is increased with concomitant use of gemfibrozil with statins, including LIVALO. Intervention: Avoid concomitant use of gemfibrozil with LIVALO. Erythromycin Clinical Impact: Erythromycin significantly increases pitavastatin exposure and increases the risk of myopathy and rhabdomyolysis. Intervention: In patients taking erythromycin, do not exceed LIVALO 1 mg once daily [see Dosage and Administration (2.4)]. Rifampin Clinical Impact: Rifampin significantly increases peak pitavastatin exposure and increases the risk of myopathy and rhabdomyolysis. Intervention: In patients taking rifampin, do not exceed LIVALO 2 mg once daily [see Dosage and Administration (2.4)]. Fibrates Clinical Impact: Fibrates may cause myopathy when given alone. The risk of myopathy and rhabdomyolysis is increased with concomitant use of fibrates with statins, including LIVALO. Intervention: Consider if the benefit of using fibrates concomitantly with LIVALO outweighs the increased risk of myopathy and rhabdomyolysis. Niacin Clinical Impact: The risk of myopathy and rhabdomyolysis may be increased with concomitant use of lipid-modifying doses (≥1 g/day) of niacin with LIVALO. Intervention: Consider if the benefit of using lipid-modifying doses (≥1 g/day) of niacin concomitantly with LIVALO outweighs the increased risk of myopathy and rhabdomyolysis. Colchicine Clinical Impact: Cases of myopathy and rhabdomyolysis have been reported with concomitant use of colchicine with statins, including LIVALO. Intervention: Consider the risk/benefit of concomitant use of colchicine with LIVALO. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
LIVALO is contraindicated for use in pregnant women since safety in pregnant women has not been established and there is no apparent benefit to therapy with LIVALO during pregnancy. Because HMG-CoA reductase inhibitors decrease cholesterol synthesis and possibly the synthesis of other biologically active substances derived from cholesterol, LIVALO may cause fetal harm when administered to pregnant women. LIVALO should be discontinued as soon as pregnancy is recognized [see Contraindications (4)]. Limited published data on the use of LIVALO are insufficient to determine a drug-associated risk of major congenital malformations or miscarriage. In animal reproduction studies, no embryo-fetal toxicity or congenital malformations were observed when pregnant rats and rabbits were orally administered pitavastatin during organogenesis at exposures which were 22 and 4 times, respectively, the maximum recommended human dose (MRHD) [see Data].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. Adverse outcomes in pregnancy occur regardless of the health of the mother or the use of medications. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Human Data
Limited published data on LIVALO have not reported a drug-associated risk of major congenital malformations or miscarriage. Rare reports of congenital anomalies have been received following intrauterine exposure to HMG-CoA reductase inhibitors. In a review of about 100 prospectively followed pregnancies in women exposed to other HMG-CoA reductase inhibitors, the incidences of congenital anomalies, spontaneous abortions, and fetal deaths/stillbirths did not exceed the rate expected in the general population. The number of cases is adequate to exclude a greater than or equal to a 3- to 4-fold increase in congenital anomalies over background incidence. In 89% of the prospectively followed pregnancies, drug treatment was initiated prior to pregnancy and was discontinued at some point in the first trimester when pregnancy was identified.
Animal Data
Reproductive toxicity studies have shown that pitavastatin crosses the placenta in rats and is found in fetal tissues at ≤36% of maternal plasma concentrations following a single dose of 1 mg/kg/day during gestation.
Embryo-fetal developmental studies were conducted in pregnant rats treated with 3, 10, 30 mg/kg/day pitavastatin by oral gavage during organogenesis. No adverse effects were observed at 3 mg/kg/day, systemic exposures 22 times human systemic exposure at 4 mg/day based on AUC.
Embryo-fetal developmental studies were conducted in pregnant rabbits treated with 0.1, 0.3, 1 mg/kg/day pitavastatin by oral gavage during the period of fetal organogenesis. Maternal toxicity consisting of reduced body weight and abortion was observed at all doses tested (4 times human systemic exposure at 4 mg/day based on AUC).
In perinatal/postnatal studies in pregnant rats given oral gavage doses of pitavastatin at 0.1, 0.3, 1, 3, 10, 30 mg/kg/day from organogenesis through weaning, maternal toxicity consisting of mortality at ≥0.3 mg/kg/day and impaired lactation at all doses contributed to the decreased survival of neonates in all dose groups (0.1 mg/kg/day represents approximately 1 time human systemic exposure at 4 mg/day dose based on AUC).
8.2 Lactation
Risk Summary
LIVALO is contraindicated during breastfeeding [see Contraindications (4)]. There is no available information on the effects of the drug on the breastfed infant or the effects of the drug on milk production. However, it has been shown that another drug in this class passes into human milk. Because of the potential for serious adverse reactions in a breastfed infant, advise patients that breastfeeding is not recommended during treatment with LIVALO.
8.3 Females and Males of Reproductive Potential
Contraception
Females
LIVALO may cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with LIVALO.
8.4 Pediatric Use
The safety and effectiveness of LIVALO as an adjunctive therapy to diet to reduce elevated TC, LDL-C, and Apo B in pediatric patients aged 8 years and older with HeFH have been established. Use of LIVALO for this indication is supported by a 12-week, double-blind, placebo-controlled trial in 82 pediatric patients 8 to 16 years of age with HeFH [see Clinical Studies (14.2)] and a 52-week open-label trial in 85 pediatric patients with HeFH.
The safety and effectiveness of LIVALO have not been established in pediatric patients younger than 8 years of age with HeFH or in pediatric patients with other types of hyperlipidemia (other than HeFH).
8.5 Geriatric Use
In controlled clinical studies, 1,209 (43%) patients were 65 years and older. No significant differences in efficacy or safety were observed between geriatric patients and younger patients.
Advanced age (≥65 years) is a risk factor for myopathy and rhabdomyolysis. In general, dose selection for a geriatric patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy and the higher risk of myopathy [see Warnings and Precautions (5.1)].
8.6 Renal Impairment
Renal impairment is a risk factor for myopathy and rhabdomyolysis. Due to the risk of myopathy, a dosage modification of LIVALO is recommended for adults with moderate and severe renal impairment (estimated glomerular filtration rate 30 – 59 mL/min/1.73 m2 and 15 – 29 mL/min/1.73 m2, respectively), as well as end-stage renal disease receiving hemodialysis. A recommended dosage for pediatric patients with renal impairment has not been established [see Dosage and Administration (2.3), Warnings and Precautions (5.1), Clinical Pharmacology (12.3)].
- 10 OVERDOSAGE
-
11 DESCRIPTION
LIVALO (pitavastatin) tablets for oral use is an HMG-CoA reductase inhibitor.
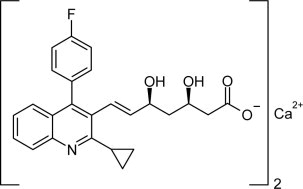
The chemical name for pitavastatin is (+)monocalcium bis{(3R, 5S, 6E)-7-[2-cyclopropyl-4-(4-fluorophenyl)-3-quinolyl]-3,5-dihydroxy-6-heptenoate}. The structural formula is:
The empirical formula for pitavastatin is C50H46CaF2N2O8 and the molecular weight is 880.98. Pitavastatin is odorless and occurs as white to pale-yellow powder. It is freely soluble in pyridine, chloroform, dilute hydrochloric acid, and tetrahydrofuran, soluble in ethylene glycol, sparingly soluble in octanol, slightly soluble in methanol, very slightly soluble in water or ethanol, and practically insoluble in acetonitrile or diethyl ether. Pitavastatin is hygroscopic and slightly unstable in light.
Each film-coated tablet of LIVALO contains 1 mg, 2 mg, or 4 mg of pitavastatin, which is equivalent to 1.045 mg, 2.09 mg, or 4.18 mg, respectively, of pitavastatin calcium and the following inactive ingredients: hypromellose, lactose monohydrate, low substituted hydroxypropylcellulose, magnesium aluminometasilicate, and magnesium stearate. The film coating contains the following inactive ingredients: colloidal anhydrous silica, hypromellose, titanium dioxide, and triethyl citrate.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Pitavastatin is an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the enzyme that catalyzes the conversion of HMG-CoA to mevalonate, a rate-limiting step in the biosynthetic pathway for cholesterol. As a result, the expression of LDL-receptors followed by the uptake of LDL from blood to liver is accelerated and then the plasma TC decreases. Sustained inhibition of cholesterol synthesis in the liver also decreases levels of very low density lipoproteins.
12.2 Pharmacodynamics
Cardiac Electrophysiology
In a randomized, double-blind, placebo-controlled, 4-way parallel, active-comparator study with moxifloxacin in 174 healthy participants, LIVALO was not associated with clinically meaningful prolongation of the QTc interval or heart rate at daily doses up to 16 mg (4 times the recommended maximum dose of 4 mg daily).
12.3 Pharmacokinetics
Absorption
Pitavastatin peak plasma concentrations are achieved about 1 hour after oral administration. Both Cmax and AUC0-inf increased in an approximately dose-proportional manner for single LIVALO doses from 1 mg to 24 mg once daily. The absolute bioavailability of pitavastatin oral solution is 51%. The Cmax and AUC of pitavastatin did not differ following evening or morning drug administration. In healthy volunteers receiving 4 mg pitavastatin, the percent change from baseline for LDL-C following evening dosing was slightly greater than that following morning dosing. Pitavastatin was absorbed in the small intestine but very little in the colon.
Effect of Food
Administration of LIVALO with a high fat meal (50% fat content) decreases pitavastatin Cmax by 43% but does not significantly reduce pitavastatin AUC.
Metabolism
The principal route of pitavastatin metabolism is glucuronidation via liver uridine 5'-diphosphate glucuronosyltransferase (UGT) with subsequent formation of pitavastatin lactone. There is only minimal metabolism by the cytochrome P450 system. Pitavastatin is marginally metabolized by CYP2C9 and to a lesser extent by CYP2C8. The major metabolite in human plasma is the lactone, which is formed via an ester-type pitavastatin glucuronide conjugate by UGTs (UGT1A3 and UGT2B7).
Excretion
A mean of 15% of radioactivity of orally administered, single 32 mg 14C-labeled pitavastatin dose was excreted in urine, whereas a mean of 79% of the dose was excreted in feces within 7 days. The mean plasma elimination half-life is approximately 12 hours.
Specific Populations
Racial or Ethnic Groups
In pharmacokinetic studies pitavastatin Cmax and AUC were 21 and 5% lower, respectively in Black or African American healthy volunteers compared with those of Caucasian healthy volunteers. In pharmacokinetic comparison between Caucasian volunteers and Japanese volunteers, there were no significant differences in Cmax and AUC.
Male and Female Patients
In a pharmacokinetic study, which compared healthy male and female volunteers, pitavastatin Cmax and AUC were 60 and 54% higher, respectively in females.
Geriatric Patients
In a pharmacokinetic study which compared healthy young and geriatric (≥65 years) volunteers, pitavastatin Cmax and AUC were 10 and 30% higher, respectively, in the geriatric patients [see Use in Specific Populations (8.5)]
Pediatric Patients
A 12-week study in pediatric patients 8 to 16 years of age treated with pitavastatin 1 mg, 2 mg and 4 mg administered once daily, showed a dose-dependent increase in pitavastatin plasma concentrations at trough (for 2 mg and 4 mg doses) and 1 hour post dose. A dose-dependent increase in pitavastatin lactone plasma concentrations was observed at trough and 1 hour post dose.
Patients with Renal Impairment
In adult patients with moderate renal impairment (estimated glomerular filtration rate of 30 – 59 mL/min/1.73 m2) and end stage renal disease receiving hemodialysis, pitavastatin AUC0-inf is 102% and 86% higher than those of healthy volunteers, respectively, while pitavastatin Cmax is 60% and 40% higher than those of healthy volunteers, respectively. Patients received hemodialysis immediately before pitavastatin dosing and did not undergo hemodialysis during the pharmacokinetic study. Hemodialysis patients have 33% and 36% increases in the mean unbound fraction of pitavastatin as compared to healthy volunteers and patients with moderate renal impairment, respectively [see Use in Specific Populations (8.6)].
In another pharmacokinetic study, adult patients with severe renal impairment (estimated glomerular filtration rate 15 – 29 mL/min/1.73 m2) not receiving hemodialysis were administered a single dose of LIVALO 4 mg. The AUC0-inf and the Cmax were 36% and 18% higher, respectively, compared with those of healthy volunteers. For both patients with severe renal impairment and healthy volunteers, the mean percentage of protein-unbound pitavastatin was approximately 0.6% [see Use in Specific Populations (8.6)].
The effect of mild renal impairment on pitavastatin exposure has not been studied.
Patients with Hepatic Impairment
The disposition of pitavastatin was compared in healthy volunteers and patients with various degrees of hepatic impairment. Pitavastatin Cmax and AUCinf in patients with moderate hepatic impairment (Child-Pugh B disease) was 2.7-fold and 3.8-fold higher, respectively as compared to healthy volunteers. In patients with mild hepatic impairment (Child-Pugh A disease), pitavastatin Cmax and AUCinf were 30% and 60% higher as compared to healthy volunteers. Mean pitavastatin half-life for moderate hepatic impairment, mild hepatic impairment, and healthy volunteers were 15, 10, and 8 hours, respectively [see Contraindications (4), Warnings and Precautions (5.3)].
Drug Interaction Studies
Warfarin
The steady-state pharmacodynamics (international normalized ratio [INR] and prothrombin time [PT]) and pharmacokinetics of warfarin in healthy volunteers were unaffected by the coadministration of LIVALO 4 mg daily.
Table 3 presents the effect of coadministered drugs on pitavastatin systemic exposure:
Table 3. Effect of Coadministered Drugs on Pitavastatin Systemic Exposure *Data presented as x-fold change represent the ratio between coadministration and pitavastatin alone (i.e., 1-fold = no change). Data presented as % change represent % difference relative to pitavastatin alone (i.e., 0% = no change).
† Considered clinically significant [see Dosage and Administration (2.4),Drug Interactions (7)]
BID = twice daily; QD = once daily; LA = Long Acting
Coadministered drug Dosage regimen Change in
AUC*Change in Cmax* Cyclosporine Pitavastatin 2 mg QD for 6 days + cyclosporine 2 mg/kg on Day 6 ↑ 4.6 fold † ↑ 6.6 fold † Erythromycin Pitavastatin 4 mg single dose on Day 4 + erythromycin 500 mg 4 times daily for 6 days ↑ 2.8 fold † ↑ 3.6 fold † Rifampin Pitavastatin 4 mg QD + rifampin 600 mg QD for 5 days ↑ 29% ↑ 2.0 fold † Atazanavir Pitavastatin 4 mg QD + atazanavir 300 mg daily for 5 days ↑ 31% ↑ 60% Darunavir/Ritonavir Pitavastatin 4mg QD on Days 1-5 and 12-16 + darunavir/ritonavir 800mg/100 mg QD on Days 6-16 ↓ 26% ↓ 4% Lopinavir/Ritonavir Pitavastatin 4 mg QD on Days 1-5 and 20-24 + lopinavir/ritonavir 400 mg/100 mg BID on Days 9 – 24 ↓ 20% ↓4 % Gemfibrozil Pitavastatin 4 mg QD + gemfibrozil 600 mg BID for 7 days ↑ 45% ↑ 31% Fenofibrate Pitavastatin 4 mg QD + fenofibrate 160 mg QD for 7 days ↑18% ↑ 11% Ezetimibe Pitavastatin 2 mg QD + ezetimibe 10 mg for 7 days ↓ 2% ↓0.2% Enalapril Pitavastatin 4 mg QD + enalapril 20 mg daily for 5 days ↑ 6% ↓ 7% Digoxin Pitavastatin 4 mg QD + digoxin 0.25 mg for 7 days ↑ 4% ↓ 9% Diltiazem LA Pitavastatin 4 mg QD on Days 1-5 and 11-15 and diltiazem LA 240 mg on Days 6-15 ↑10% ↑15% Grapefruit Juice Pitavastatin 2 mg single dose on Day 3 + grapefruit juice for 4 days ↑ 15% ↓ 12% Itraconazole Pitavastatin 4 mg single dose on Day 4 + itraconazole 200 mg daily for 5 days ↓ 23% ↓ 22% Table 4 presents the effect of pitavastatin coadministration on systemic exposure of other drugs:
Table 4. Effect of Pitavastatin Coadministration on Systemic Exposure to Other Drugs *Data presented as % change represent % difference relative to the investigated drug alone
(i.e., 0% = no change).BID = twice daily; QD = once daily; LA = Long Acting
Coadministered drug Dosage regimen Change in
AUC*Change in Cmax* Atazanavir Pitavastatin 4 mg QD + atazanavir 300 mg daily for 5 days ↑ 6% ↑ 13% Darunavir Pitavastatin 4mg QD on Days 1-5 and 12-16 + darunavir/ritonavir 800mg/100 mg QD on Days 6-16 ↑ 3% ↑ 6% Lopinavir Pitavastatin 4 mg QD on Days 1-5 and 20-24 + lopinavir/ritonavir 400 mg/100 mg BID on Days 9 – 24 ↓ 9% ↓ 7% Ritonavir Pitavastatin 4 mg QD on Days 1-5 and 20-24 + lopinavir/ritonavir 400 mg/100 mg BID on Days 9 – 24 ↓ 11% ↓ 11% Ritonavir Pitavastatin 4mg QD on Days 1-5 and 12-16 + darunavir/ritonavir 800mg/100 mg QD on Days 6-16 ↑ 8% ↑ 2% Enalapril Pitavastatin 4 mg QD + enalapril 20 mg
daily for 5 daysEnalapril ↑ 12% ↑ 12% Enalaprilat ↓ 1% ↓ 1% Warfarin Individualized maintenance dose of warfarin (2 - 7 mg) for 8 days + pitavastatin 4 mg QD for 9 days R-warfarin ↑ 7% ↑ 3% S-warfarin ↑ 6% ↑ 3% Ezetimibe Pitavastatin 2 mg QD + ezetimibe 10 mg for 7 days ↑ 9% ↑ 2% Digoxin Pitavastatin 4 mg QD + digoxin 0.25 mg for 7 days ↓ 3% ↓ 4% Diltiazem LA Pitavastatin 4 mg QD on Days 1-5 and 11-15 and diltiazem LA 240 mg on Days 6-15 ↓ 2% ↓ 7% Rifampin Pitavastatin 4 mg QD + rifampin 600 mg QD for 5 days ↓ 15% ↓ 18% -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 92-week carcinogenicity study in mice given pitavastatin, at the maximum tolerated dose of 75 mg/kg/day with systemic maximum exposures (AUC) 26 times the clinical maximum exposure at 4 mg daily, there was an absence of drug-related tumors.
In a 92-week carcinogenicity study in rats given pitavastatin at 1, 5, 25 mg/kg/day by oral gavage there was a significant increase in the incidence of thyroid follicular cell tumors at 25 mg/kg/day, which represents 295 times human systemic exposures based on AUC at the 4 mg daily maximum human dose.
In a 26-week transgenic mouse (Tg rasH2) carcinogenicity study where animals were given pitavastatin at 30, 75, and 150 mg/kg/day by oral gavage, no clinically significant tumors were observed.
Pitavastatin was not mutagenic in the Ames test with Salmonella typhimurium and Escherichia coli with and without metabolic activation, the micronucleus test following a single administration in mice and multiple administrations in rats, the unscheduled DNA synthesis test in rats, and a Comet assay in mice. In the chromosomal aberration test, clastogenicity was observed at the highest doses tested, which also elicited high levels of cytotoxicity.
Pitavastatin had no adverse effects on male and female rat fertility at oral doses of 10 and 30 mg/kg/day, respectively, at systemic exposures 56- and 354-times clinical exposure at 4 mg daily based on AUC.
Pitavastatin treatment in rabbits resulted in mortality in males and females given 1 mg/kg/day (30-times clinical systemic exposure at 4 mg daily based on AUC) and higher during a fertility study. Although the cause of death was not determined, rabbits had gross signs of renal toxicity (kidneys whitened) indicative of possible ischemia. Lower doses (15-times human systemic exposure) did not show significant toxicity in adult males and females. However, decreased implantations, increased resorptions, and decreased viability of fetuses were observed.
13.2 Animal Toxicology and/or Pharmacology
Central Nervous System Toxicity
CNS vascular lesions, characterized by perivascular hemorrhages, edema, and mononuclear cell infiltration of perivascular spaces, have been observed in dogs treated with several other members of this drug class. A chemically similar drug in this class produced dose-dependent optic nerve degeneration (Wallerian degeneration of retinogeniculate fibers) in dogs, at a dose that produced plasma drug levels about 30 times higher than the mean drug level in humans taking the highest recommended dose. Wallerian degeneration has not been observed with pitavastatin. Cataracts and lens opacities were seen in dogs treated for 52 weeks at a dose level of 1 mg/kg/day (9 times clinical exposure at the maximum human dose of 4 mg/day based on AUC comparisons).
-
14 CLINICAL STUDIES
14.1 Primary Hyperlipidemia or Mixed Dyslipidemia in Adult Patients
Active-Controlled Study with Atorvastatin (Study 301)
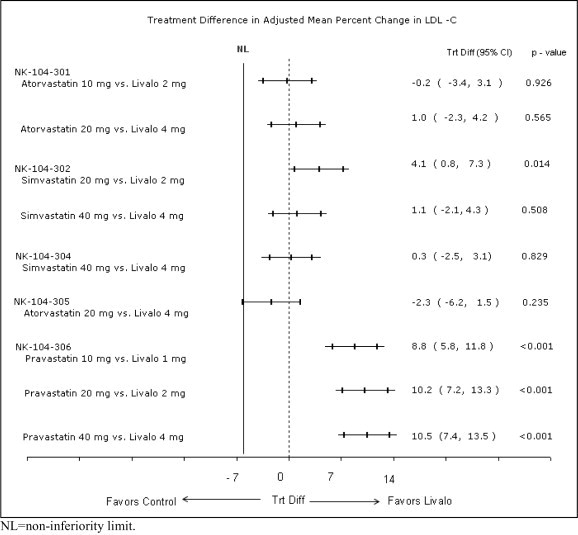
LIVALO was compared with Atorvastatin Calcium Tablets (referred to as atorvastatin) in a randomized, multicenter, double-blind, double-dummy, active-controlled, non-inferiority study of 817 adult patients with primary hyperlipidemia or mixed dyslipidemia. Patients entered a 6- to 8-week wash-out/dietary lead-in period and then were randomized to a 12-week treatment with either LIVALO or atorvastatin (Table 5). Non-inferiority of pitavastatin to a given dose of atorvastatin was considered to be demonstrated if the lower bound of the 95% CI for the mean treatment difference was greater than -6% for the mean percent change in LDL-C.
Lipid results are shown in Table 5. For the percent change from baseline to endpoint in LDL-C, LIVALO was non-inferior to atorvastatin for the two pairwise comparisons: LIVALO 2 mg vs. atorvastatin 10 mg and LIVALO 4 mg vs. atorvastatin 20 mg. Mean treatment differences (95% CI) were 0% (-3%, 3%) and 1% (-2%, 4%), respectively.
Table 5. Lipid Response by Dose of LIVALO and Atorvastatin in Adult Patients with Primary Hyperlipidemia or Mixed Dyslipidemia in Study 301 (Mean % Change from Baseline at Week 12) Treatment N LDL-C Apo-B TC TG HDL-C non-HDL-C LIVALO
2 mg daily315 -38 -30 -28 -14 4 -35 LIVALO
4 mg daily298 -45 -35 -32 -19 5 -41 Atorvastatin
10 mg daily102 -38 -29 -28 -18 3 -35 Atorvastatin
20 mg daily102 -44 -36 -33 -22 2 -41 Active-Controlled Study with Simvastatin (Study 302)
LIVALO was compared with Simvastatin Tablets (referred to as simvastatin) in a randomized, multicenter, double-blind, double-dummy, active-controlled, non-inferiority study of 843 adult patients with primary hyperlipidemia or mixed dyslipidemia. Patients entered a 6- to 8-week wash-out/dietary lead-in period and then were randomized to a 12 week treatment with either LIVALO or simvastatin (Table 6). Non-inferiority of pitavastatin to a given dose of simvastatin was considered to be demonstrated if the lower bound of the 95% CI for the mean treatment difference was greater than -6% for the mean percent change in LDL-C.
Lipid results are shown in Table 6. For the percent change from baseline to endpoint in LDL-C, LIVALO was non-inferior to simvastatin for the two pairwise comparisons: LIVALO 2 mg vs. simvastatin 20 mg and LIVALO 4 mg vs. simvastatin 40 mg. Mean treatment differences (95% CI) were 4% (1%, 7%) and 1% (-2%, 4%), respectively.
Table 6. Lipid Response by Dose of LIVALO and Simvastatin in Adult Patients with Primary Hyperlipidemia or Mixed Dyslipidemia in Study 302 (Mean % Change from Baseline at Week 12) Treatment N LDL-C Apo-B TC TG HDL-C non-HDL-C LIVALO
2 mg daily307 -39 -30 -28 -16 6 -36 LIVALO
4 mg daily319 -44 -35 -32 -17 6 -41 Simvastatin
20 mg daily107 -35 -27 -25 -16 6 -32 Simvastatin
40 mg daily110 -43 -34 -31 -16 7 -39 Active-Controlled Study with Pravastatin in Geriatric Patients (Study 306)
LIVALO was compared with Pravastatin Sodium Tablets (referred to as pravastatin) in a randomized, multicenter, double-blind, double-dummy, parallel group, active-controlled non-inferiority study of 942 geriatric patients (≥65 years) with primary hyperlipidemia or mixed dyslipidemia. Patients entered a 6- to 8-week wash-out/dietary lead-in period, and then were randomized to a once daily dose of LIVALO or pravastatin for 12 weeks (Table 7). Non-inferiority of LIVALO to a given dose of pravastatin was assumed if the lower bound of the 95% CI for the treatment difference was greater than -6% for the mean percent change in LDL-C.
Lipid results are shown in Table 7. LIVALO significantly reduced LDL-C compared to pravastatin as demonstrated by the following pairwise dose comparisons: LIVALO 1 mg vs. pravastatin 10 mg, LIVALO 2 mg vs. pravastatin 20 mg and LIVALO 4 mg vs. pravastatin 40 mg. Mean treatment differences (95% CI) were 9% (6%, 12%), 10% (7%, 13%) and 10% (7%, 13% ), respectively.
Table 7. Lipid Response by Dose of LIVALO and Pravastatin in Geriatric Patients with Primary Hyperlipidemia or Mixed Dyslipidemia in Study 306 (Mean % Change from Baseline at Week 12) Treatment N LDL-C Apo-B TC TG HDL-C non-HDL-C LIVALO
1 mg daily207 -31 -25 -22 -13 1 -29 LIVALO
2 mg daily224 -39 -31 -27 -15 2 -36 LIVALO
4 mg daily210 -44 -37 -31 -22 4 -41 Pravastatin
10 mg daily103 -22 -17 -15 -5 0 -20 Pravastatin
20 mg daily96 -29 -22 -21 -11 -1 -27 Pravastatin
40 mg daily102 -34 -28 -24 -15 1 -32 Active-Controlled Study with Simvastatin in Patients with ≥2 Risk Factors for Coronary Heart Disease (Study 304)
LIVALO was compared with Simvastatin Tablets (referred to as simvastatin) in a randomized, multicenter, double-blind, double-dummy, active-controlled, non-inferiority study of 351 adult patients with primary hyperlipidemia or mixed dyslipidemia with ≥2 risk factors for coronary heart disease. After a 6- to 8-week wash-out/dietary lead-in period, patients were randomized to a 12-week treatment with either LIVALO or simvastatin (Table 8). Non-inferiority of LIVALO to simvastatin was considered to be demonstrated if the lower bound of the 95% CI for the mean treatment difference was greater than -6% for the mean percent change in LDL-C.
Lipid results are shown in Table 8. LIVALO 4 mg was non-inferior to simvastatin 40 mg for percent change from baseline to endpoint in LDL-C. The mean treatment difference (95% CI) was 0% (-2%, 3%).
Table 8. Lipid Response by Dose of LIVALO and Simvastatin in Adult Patients with Primary Hyperlipidemia or Mixed Dyslipidemia with ≥2 Risk Factors for Coronary Heart Disease in Study 304 (Mean % Change from Baseline at Week 12) Treatment N LDL-C Apo-B TC TG HDL-C non-HDL-C LIVALO
4 mg daily233 -44 -34 -31 -20 7 -40 Simvastatin
40 mg daily118 -44 -34 -31 -15 5 -39 Active-Controlled Study with Atorvastatin in Patients with Type 2 Diabetes Mellitus (Study 305)
LIVALO was compared with Atorvastatin Calcium Tablets (referred to as atorvastatin) in a randomized, multicenter, double-blind, double-dummy, parallel group, active-controlled, non-inferiority study of 410 adult patients with type 2 diabetes mellitus and mixed dyslipidemia. Patients entered a 6- to 8-week washout/dietary lead-in period and were randomized to a once daily dose of LIVALO or atorvastatin for 12 weeks. Non-inferiority of LIVALO was considered to be demonstrated if the lower bound of the 95% CI for the mean treatment difference was greater than -6% for the mean percent change in LDL-C.
Lipid results are shown in Table 9. The treatment difference (95% CI) for LDL-C percent change from baseline was -2% (-6.2%, 1.5%). The two treatment groups were not statistically different on LDL-C. However, the lower limit of the CI was -6.2%, slightly exceeding the -6% non-inferiority limit. The study failed to demonstrate that LIVALO was not significantly different than atorvastatin in lowering LDL-C in patients with type 2 diabetes mellitus and mixed dyslipidemia.
Table 9. Lipid Response by Dose of LIVALO and Atorvastatin in Adult Patients with Type 2 Diabetes Mellitus and Mixed Dyslipidemia in Study 305 (Mean % Change from Baseline at Week 12) Treatment N LDL-C Apo-B TC TG HDL-C non-HDL-C LIVALO
4 mg daily274 -41 -32 -28 -20 7 -36 Atorvastatin
20 mg daily136 -43 -34 -32 -27 8 -40 The treatment differences in efficacy in LDL-C change from baseline between LIVALO and active controls (i.e., atorvastatin, simvastatin, or pravastatin) in the in the active-controlled studies described above are summarized in Figure 1.
Figure 1. Treatment Difference in Adjusted Mean Percent Change in LDL-C between LIVALO and the Comparator (Atorvastatin, Simvastatin, or Pravastatin)
14.2 HeFH in Pediatric Patients
In a double-blind, placebo-controlled, 12-week trial, 82 pediatric patients (36 boys and 46 girls), 8 to 16 years of age with genetically confirmed HeFH, fasting low-density lipoprotein cholesterol (LDL-C) ≥190 mg/dL or LDL-C ≥160 mg/dL with an additional cardiovascular risk factor (male gender, a family history of premature CV disease, presence of low HDL (<45 mg/dL) or high TG (>150 mg/dL), presence of high lipoprotein (a) (>75 nmol/L), presence of type 2 diabetes mellitus or presence of hypertension) were randomized to LIVALO 1 mg, 2 mg, and 4 mg. Mean LDL-C at baseline was 235 mg/dL (range 160.5 mg/dL to 441mg/dL). Approximately 39% of patients were Tanner Stage 1 at baseline.
LIVALO significantly reduced plasma LDL-C, non-HDL-C, TC, and Apo-B compared to placebo. The reductions in LDL-C, Apo-B, TC, and non-HDL-C were dose dependent. There was no statistically significant improvement in HDL-C or TG at any LIVALO dose. See the lipid results in Table 10.
Table 10. Lipid Response in Pediatric Patients with HeFH (Mean % Change from Baseline at Week 12) *Difference from placebo not statistically significant
# Median Percent Change from Baseline at Week 12
Treatment N LDL-C Apo-B TC TG*# HDL-C* non-HDL-C Placebo 19 -1 -3 -1 -3 -1 -1 LIVALO
1 mg daily20 -21 -20 -16 -14 7 -21 LIVALO
2 mg daily24 -30 -25 -25 -15 -3 -29 LIVALO
4 mg daily19 -38 -28 -30 5 -2 -36 The long-term efficacy of LIVALO initiated in childhood to reduce morbidity and mortality in adulthood has not been established.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
LIVALO tablets are supplied as follows:
Tablet Strength Package Size Tablet Description NDC 1 mg Bottle of 90 Round white film-coated tablet debossed “KC” on one face and “1” on the reverse 66869-104-90 2 mg Bottle of 90 Round white film-coated tablet debossed “KC” on one face and “2” on the reverse 66869-204-90 4 mg Bottle of 90 Round white film-coated tablet debossed “KC” on one face and “4” on the reverse 66869-404-90 Store at room temperature between 15°C and 30°C (59° to 86° F) [see USP]. Protect from light.
-
17 PATIENT COUNSELING INFORMATION
The patient should be informed of the following:
Myopathy and Rhabdomyolysis
Advise patients that LIVALO may cause myopathy and rhabdomyolysis. Inform patients that the risk is increased when taking certain types of medication and they should discuss all medication, both prescription and over the counter, with their healthcare provider. Instruct patients to promptly report any unexplained muscle pain, tenderness or weakness particularly if accompanied by malaise or fever [see Warnings and Precautions (5.1)].
Hepatic Dysfunction
Inform patients that LIVALO may cause liver enzyme elevations and possibly liver failure. Advise patients to promptly report fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice [see Warnings and Precautions (5.3)].
Increases in HbA1c and Fasting Serum Glucose Levels
Inform patients that increases in HbA1c and fasting serum glucose levels may occur with LIVALO. Encourage patients to optimize lifestyle measures, including regular exercise, maintaining a healthy body weight, and making healthy food choices [see Warnings and Precautions (5.4)].
Embryo-fetal Toxicity
Advise females of reproductive potential of the potential risk to a fetus, to use effective contraception during treatment and to inform their healthcare professional of a known or suspected pregnancy [see Contraindications (4), Use in Specific Populations (8.1, 8.3)].
Lactation
Advise women not to breastfeed during treatment with LIVALO [see Contraindications (4), Use in Specific Populations (8.2)].
Liver Enzymes
It is recommended that liver enzyme tests be checked before the initiation of LIVALO and if signs or symptoms of liver injury occur. All patients treated with LIVALO should be advised to report promptly any symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice.
Manufactured for:
Kowa Company, Limited Tokyo 103-8433 Japan
Manufactured by:
Patheon Pharmaceuticals, Inc. Cincinnati, OH 45237 USA or by Kowa Company, LTD Nagoya, 462-0024 Japan
Marketed and Distributed by:
Kowa Pharmaceuticals America, Inc. Montgomery, AL 36117 USALIVALO is a trademark of the Kowa group of companies.
© Kowa Pharmaceuticals America, Inc. (2009) -
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Bottle Label Livalo 1 mg
NDC: 66869-104-90
Livalo ®
(pitavastatin) tablets1 mg*
Rx Only
LOGO-Kowa-LOGO

-
PRINCIPAL DISPLAY PANEL
Principal Display Panel - Bottle Label Livalo 2 mg
NDC: 66869-204-90
Livalo®
(pitavastatin) tablets2 mg*
Rx Only
LOGO-Kowa-LOGO

-
PRINCIPAL DISPLAY PANEL
Principal display Panel - Bottle Label Livalo 4 mg
NDC: 66869-404-90
Livalo®
(pitavastatin) tablets4 mg*
Rx Only
LOGO-Kowa-LOGO

-
PRINCIPAL DISPLAY PANEL
Principal display Panel – Carton 2 mg
FREE SAMPLE NDC: 66869-204-07
Livalo ®
(pitavastatin) tablets
2 mg*
Rx Only
Contains 7 Tablets
LOGO-Kowa-LOGO
Professional Sample –
Not for Sale
*Each tablet contains:
Active ingredient: pitavastatin calcium 2.09 mg equivalent to pitavastatin 2 mg.
-
PRINCIPAL DISPLAY PANEL
Principal display Panel – Carton 4 mg
FREE SAMPLE NDC: 66869-404-07
Livalo ®
(pitavastatin) tablets4 mg*
Rx Only
Contains 7 Tablets
LOGO-Kowa-LOGO
Professional Sample –
Not for Sale*Each tablet contains:
Active ingredient: pitavastatin calcium 4.18 mg equivalent to pitavastatin 4 mg.
-
INGREDIENTS AND APPEARANCE
LIVALO
pitavastatin calcium tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66869-104 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength pitavastatin calcium (UNII: IYD54XEG3W) (pitavastatin - UNII:M5681Q5F9P) pitavastatin 1.045 mg Inactive Ingredients Ingredient Name Strength lactose monohydrate (UNII: EWQ57Q8I5X) hydroxypropyl cellulose, low substituted (UNII: 2165RE0K14) hypromellose, unspecified (UNII: 3NXW29V3WO) magnesium aluminometasilicate type i-b (UNII: 8XK1039013) magnesium stearate (UNII: 70097M6I30) titanium dioxide (UNII: 15FIX9V2JP) triethyl citrate (UNII: 8Z96QXD6UM) silicon dioxide (UNII: ETJ7Z6XBU4) Product Characteristics Color white (WHITE) Score no score Shape ROUND (ROUND) Size 6mm Flavor Imprint Code 1;KC Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66869-104-90 90 in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/16/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022363 05/16/2019 LIVALO
pitavastatin calcium tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66869-204 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength pitavastatin calcium (UNII: IYD54XEG3W) (pitavastatin - UNII:M5681Q5F9P) pitavastatin 2.09 mg Inactive Ingredients Ingredient Name Strength lactose monohydrate (UNII: EWQ57Q8I5X) hydroxypropyl cellulose, low substituted (UNII: 2165RE0K14) hypromellose, unspecified (UNII: 3NXW29V3WO) magnesium aluminometasilicate type i-b (UNII: 8XK1039013) magnesium stearate (UNII: 70097M6I30) titanium dioxide (UNII: 15FIX9V2JP) triethyl citrate (UNII: 8Z96QXD6UM) silicon dioxide (UNII: ETJ7Z6XBU4) Product Characteristics Color white (WHITE) Score no score Shape ROUND (ROUND) Size 8mm Flavor Imprint Code 2;KC Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66869-204-90 90 in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/16/2019 2 NDC: 66869-204-07 7 in 1 CARTON; Type 0: Not a Combination Product 05/16/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022363 05/16/2019 LIVALO
pitavastatin calcium tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 66869-404 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength pitavastatin calcium (UNII: IYD54XEG3W) (pitavastatin - UNII:M5681Q5F9P) pitavastatin 4.18 mg Inactive Ingredients Ingredient Name Strength lactose monohydrate (UNII: EWQ57Q8I5X) hydroxypropyl cellulose, low substituted (UNII: 2165RE0K14) hypromellose, unspecified (UNII: 3NXW29V3WO) magnesium aluminometasilicate type i-b (UNII: 8XK1039013) magnesium stearate (UNII: 70097M6I30) titanium dioxide (UNII: 15FIX9V2JP) triethyl citrate (UNII: 8Z96QXD6UM) silicon dioxide (UNII: ETJ7Z6XBU4) Product Characteristics Color white (WHITE) Score no score Shape ROUND (ROUND) Size 10mm Flavor Imprint Code 4;KC Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 66869-404-90 90 in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 05/16/2019 2 NDC: 66869-404-07 7 in 1 CARTON; Type 0: Not a Combination Product 05/16/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022363 05/16/2019 Labeler - Kowa Pharmaceuticals America, Inc. (119459589) Establishment Name Address ID/FEI Business Operations Kowa Company, Ltd. 717190628 MANUFACTURE(66869-104, 66869-204, 66869-404)
Trademark Results [Livalo]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LIVALO 79016899 3228048 Live/Registered |
KOWA COMPANY, LTD. 2005-08-26 |
 LIVALO 76600233 2993941 Dead/Cancelled |
Livalo Design Inc. 2004-07-01 |
 LIVALO 76147782 not registered Dead/Abandoned |
Livalo Design Inc. 2000-10-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.