CLINDAMYCIN PHOSPHATE injection, solution
clindamycin phosphate by
Drug Labeling and Warnings
clindamycin phosphate by is a Prescription medication manufactured, distributed, or labeled by Baxter Healthcare Corporation. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CLINDAMYCIN IN 0.9% SODIUM CHLORIDE injection safely and effectively. See full prescribing information for CLINDAMYCIN IN 0.9% SODIUM CHLORIDE injection.
CLINDAMYCIN IN 0.9% SODIUM CHLORIDE injection, for intravenous use
Initial U.S. Approval: 1989WARNING: CLOSTRIDIUM DIFFICILE-ASSOCIATED DIARRHEA (CDAD) and COLITIS
See full prescribing information for complete boxed warning.
Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Clindamycin in 0.9% Sodium Chloride Injection and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile (5.1).
Because Clindamycin in 0.9% Sodium Chloride Injection therapy has been associated with severe colitis which may end fatally, it should be reserved for serious infections where less toxic antimicrobial agents are inappropriate (1). It should not be used in patients with nonbacterial infections such as most upper respiratory tract infections. C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy (5.1).
INDICATIONS AND USAGE
Clindamycin is a lincosamide antibacterial indicated for the treatment of the following:
- Serious infections caused by susceptible anaerobic bacteria (1.1)
- Infections Due to Susceptible Strains of Streptococci, Pneumococci and Staphylococci. (1.2)
- Lower Respiratory Tract Infections. (1.3)
- Skin and Skin Structure Infections. (1.4)
- Gynecological Infections. (1.5)
- Intra-abdominal Infections. (1.6)
- Septicemia. (1.7)
- Bone and Joint Infections. (1.8)
Limitation of use
Since clindamycin does not diffuse adequately into the cerebrospinal fluid, Clindamycin in 0.9% Sodium Chloride Injection should not be used in the treatment of meningitis (1.9)To reduce the development of drug-resistant bacteria and maintain the effectiveness of Clindamycin in 0.9% Sodium Chloride Injection and other antibacterial drugs, Clindamycin in 0.9% Sodium Chloride Injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. (1.10)
DOSAGE AND ADMINISTRATION
- Recommended Adult Dosage: Serious infections due to aerobic gram-positive cocci and the more susceptible anaerobes (NOT generally including Bacteroides fragilis, Peptococcus species and Clostridium species other than Clostridium perfringens): 600–1200 mg/day in 2, 3 or 4 equal doses by intravenous infusion. (2.2)
- More severe infections, particularly those due to proven or suspected Bacteroides fragilis, Peptococcus species, or Clostridium species other than Clostridium perfringens: 1200–2700 mg/day in 2, 3 or 4 equal doses by intravenous infusion. (2.2)
- Dosage in Pediatric Patients (1 Month of Age to 16 Years): 20 to 40 mg/kg/day in 3 or 4 equal doses by intravenous infusion. (2.3)
- Alternative Pediatric Patients Dosing: 350 mg/m2/day for serious infections and 450 mg/m2/day for more severe infections. (2.3)
- Dosage in Neonates (Less than 1 Month of Age): 15 to 20 mg/kg/day in 3 to 4 equal doses by intravenous infusion. (2.4)
DOSAGE FORMS AND STRENGTHS
Each 50 mL of Clindamycin in 0.9% Sodium Chloride Injection, 300 mg/50 mL (6 mg/mL), 600 mg/50 mL (12 mg/mL), and 900 mg/50 mL (18 mg/mL) contains 300 mg, 600 mg, or 900 mg clindamycin, respectively (as clindamycin phosphate, USP), in a single-dose GALAXY container. (3)
CONTRAINDICATIONS
Individuals with a history of hypersensitivity to preparations containing clindamycin or lincomycin. (4)
WARNINGS AND PRECAUTIONS
- Anaphylactic shock and anaphylactic reactions have been reported. (5.2)
- Elderly patients with associated severe illness may have a greater risk of developing adverse reactions from diarrhea. (5.3)
- Clindamycin in 0.9% Sodium Chloride Injection products should be avoided in individuals with a history of gastrointestinal disease, particularly colitis. (5.4)
- Clindamycin in 0.9% Sodium Chloride Injection should be avoided in atopic individuals. (5.5)
- During prolonged therapy periodic liver and kidney function tests and blood counts should be performed. (5.6)
- The use of Clindamycin in 0.9% Sodium Chloride Injection may result in overgrowth of nonsusceptible organisms-particularly yeasts. (5.7)
- Prescribing Clindamycin in 0.9% Sodium Chloride Injection in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria. (5.8)
ADVERSE REACTIONS
Most common adverse reactions: gastrointestinal (abdominal pain, nausea, vomiting) and hypersensitivity reactions (anaphylaxis, urticaria, skin rash). (6)
To report SUSPECTED ADVERSE REACTIONS, contact Baxter Healthcare at 1-866-888-2472 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Neuromuscular blocking properties that may enhance the action of other neuromuscular blocking agents. (7.1)
- Monitor for adverse reactions when strong CYP3A4 and/or CYP3A5 inhibitors are coadministered with clindamycin. (7.2)
- In the presence of strong CYP3A4 and/or CYP3A5 inducers such as rifampicin, monitor for loss of effectiveness. (7.3)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: CLOSTRIDIUM DIFFICILE-ASSOCIATED DIARRHEA (CDAD) and COLITIS
1 INDICATIONS AND USAGE
1.1 Infections Due to Susceptible Anaerobic Bacteria
1.2 Infections Due to Susceptible Strains of Streptococci, Pneumococci and Staphylococci
1.3 Lower Respiratory Tract Infections
1.4 Skin and Skin Structure Infections
1.5 Gynecological Infections
1.6 Intra-abdominal Infections
1.7 Septicemia
1.8 Bone and Joint Infections
1.9 Limitations of Use
1.10 Usage
2 DOSAGE AND ADMINISTRATION
2.1 Important Discontinuation Instructions
2.2 Recommended Adult Dosage
2.3 Dosage in Pediatric Patients (1 Month of Age to 16 Years)
2.4 Dosage in Pediatric Patients (Neonates Less than 1 Month)
2.5 Intravenous Infusion Rates
2.6 Directions for Use of Clindamycin in 0.9% Sodium Chloride Injection in GALAXY Plastic Container
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Clostridium difficile Associated Diarrhea
5.2 Anaphylactic and Severe Hypersensitivity Reactions
5.3 Diarrhea in Elderly Patients with Associated Severe Illness
5.4 Use in Patients with Gastrointestinal Disease
5.5 Use in Atopic Individuals
5.6 Laboratory Tests: Monitoring to Assess Safety
5.7 Overgrowth of Nonsusceptible Organisms
5.8 Development of Drug-Resistant Bacteria
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Neuromuscular Blocking Agents
7.2 Inhibitors of CYP3A4 and CYP3A5
7.3 Inhibitors of CYP3A4 and CYP3A5
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: CLOSTRIDIUM DIFFICILE-ASSOCIATED DIARRHEA (CDAD) and COLITIS
Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Clindamycin in 0.9% Sodium Chloride Injection and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
Because Clindamycin in 0.9% Sodium Chloride Injection therapy has been associated with severe colitis which may end fatally, it should be reserved for serious infections where less toxic antimicrobial agents are inappropriate [see Indications and Usage (1)]. It should not be used in patients with nonbacterial infections such as most upper respiratory tract infections. C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated. [see Warnings and Precautions (5.1)]
-
1 INDICATIONS AND USAGE
1.1 Infections Due to Susceptible Anaerobic Bacteria
Clindamycin in 0.9% Sodium Chloride Injection is indicated for the treatment of serious infections caused by susceptible anaerobic bacteria [see Indications and Usage (1.3 - 1.7)].
1.2 Infections Due to Susceptible Strains of Streptococci, Pneumococci and Staphylococci
Clindamycin in 0.9% Sodium Chloride Injection is indicated for the treatment of serious infections due to susceptible strains of streptococci, pneumococci, and staphylococci. Its use should be reserved for penicillin-allergic patients or other patients for whom, in the judgment of the physician, a penicillin is inappropriate. Because of the risk of antibacterial-associated pseudomembranous colitis, [see Boxed Warning], before selecting clindamycin the physician should consider the nature of the infection and the suitability of less toxic alternatives (e.g., erythromycin).
Bacteriologic studies should be performed to determine the causative organisms and their susceptibility to clindamycin.
Indicated surgical procedures should be performed in conjunction with antibacterial therapy.
1.3 Lower Respiratory Tract Infections
Clindamycin in 0.9% Sodium Chloride Injection is indicated in the treatment of serious lower respiratory tract infections including pneumonia, empyema, and lung abscess caused by susceptible strains of anaerobes, Streptococcus pneumoniae, other streptococci (except E. faecalis), and Staphylococcus aureus.
1.4 Skin and Skin Structure Infections
Clindamycin in 0.9% Sodium Chloride Injection is indicated in the treatment of serious skin and skin structure infections caused by susceptible strains of Streptococcus pyogenes, Staphylococcus aureus, and anaerobes.
1.5 Gynecological Infections
Clindamycin in 0.9% Sodium Chloride Injection is indicated in the treatment of serious gynecological infections including endometritis, nongonococcal tubo-ovarian abscess, pelvic cellulitis, and postsurgical vaginal cuff infection caused by susceptible anaerobes.
1.6 Intra-abdominal Infections
Clindamycin in 0.9% Sodium Chloride Injection is indicated in the treatment of serious intra-abdominal infections including peritonitis and intra-abdominal abscess caused by susceptible anaerobic organisms.
1.7 Septicemia
Clindamycin in 0.9% Sodium Chloride Injection is indicated in the treatment of serious septicemia caused by susceptible strains of Staphylococcus aureus, streptococci (except Enterococcus faecalis), and susceptible anaerobes.
1.8 Bone and Joint Infections
Clindamycin in 0.9% Sodium Chloride Injection is indicated in the treatment of serious bone and joint infections including acute hematogenous osteomyelitis caused by susceptible strains of Staphylococcus aureus and as adjunctive therapy in the surgical treatment of chronic bone and joint infections due to susceptible organisms.
1.9 Limitations of Use
Since clindamycin does not diffuse adequately into the cerebrospinal fluid, Clindamycin in 0.9% Sodium Chloride Injection should not be used in the treatment of meningitis [see Clinical Pharmacology (12.3)].
1.10 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Clindamycin in 0.9% Sodium Chloride Injection and other antibacterial drugs, Clindamycin in 0.9% Sodium Chloride Injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Discontinuation Instructions
Discontinue Clindamycin in 0.9% Sodium Chloride Injection if diarrhea occurs during therapy [see Boxed Warning].
2.2 Recommended Adult Dosage
The recommended dosing regimen for Clindamycin in 0.9% Sodium Chloride Injection is 600-1200 mg/day in 2, 3, or 4 equal doses by intravenous infusion for serious infections due to aerobic gram-positive cocci and the more susceptible anaerobes (NOT generally including Bacteroides fragilis, Peptococcus species and Clostridium species other than Clostridium perfringens).
For more severe infections, particularly those due to proven or suspected Bacteroides fragilis, Peptococcus species, or Clostridium species other than Clostridium perfringens, the recommended dosing regimen is 1200-2700 mg/day in 2, 3, or 4 equal doses by intravenous infusion.
In life-threatening situations, these doses may be increased. Doses of as much as 4800 mg daily have been given intravenously to adults [see Dosage and Administration (2.4)].
Alternatively, drug may be administered in the form of a single rapid infusion of the first dose followed by continuous intravenous infusion as follows:
Table 1: Infusion Rates for the Administration of Clindamycin Injection by Single Rapid Infuion followed by Continuous Intravenous Infusion To maintain serum
clindamycin levelsRapid infusion rate
Maintenance infusion rate
Above 4 mcg/mL
10 mg/min for 30 min
0.75 mg/min
Above 5 mcg/mL
15 mg/min for 30 min
1.00 mg/min
Above 6 mcg/mL
20 mg/min for 30 min
1.25 mg/min
2.3 Dosage in Pediatric Patients (1 Month of Age to 16 Years)
The recommended dosing regimen for pediatric patients is 20 to 40 mg/kg/day in 3 or 4 equal doses by intravenous infusion. The higher doses would be used for more severe infections. As an alternative to dosing on a body weight basis, pediatric patients may be dosed on the basis of square meters body surface: 350 mg/m2/day for serious infections and 450 mg/m2/day for more severe infections.
Parenteral therapy may be changed to oral clindamycin palmitate hydrochloride powder for oral solution or clindamycin hydrochloride capsules when the condition warrants and at the discretion of the physician.
In cases of β-hemolytic streptococcal infections, treatment should be continued for at least 10 days.
2.4 Dosage in Pediatric Patients (Neonates Less than 1 Month)
The recommended dosing regimen for neonates is 15 to 20 mg/kg/day in 3 to 4 equal doses by intravenous infusion. The lower dosage may be adequate for small prematures.
2.5 Intravenous Infusion Rates
Infusion rates should NOT exceed 30 mg per minute. The usual infusion rates are as follows:
Dose
Time
300 mg
10 min
600 mg
20 min
900 mg
30 min
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
2.6 Directions for Use of Clindamycin in 0.9% Sodium Chloride Injection in GALAXY Plastic Container
Premixed Clindamycin in 0.9% Sodium Chloride Injection is for intravenous infusion using sterile equipment. Check for minute leaks prior to use by squeezing bag firmly. If leaks are found, discard solution as sterility may be impaired. Do NOT add supplementary medication. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Do NOT use unless solution is clear and seal is intact.
Do NOT use plastic containers in series connections. Such use could result in air embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is complete.
Preparation for Administration:
- 1. Suspend container from eyelet support.
- 2. Remove protector from outlet port at bottom of container.
- 3. Attach administration set. Refer to complete directions accompanying set.
-
3 DOSAGE FORMS AND STRENGTHS
Injection: Sterile, clear and colorless solution available in three strengths:
- 300 mg/50 mL (6 mg/mL): Each 50 mL single-dose GALAXY container contains 300 mg clindamycin (as clindamycin phosphate, USP) in 0.9% sodium chloride.
- 600 mg/50 mL (12 mg/mL): Each 50 mL single-dose GALAXY container contains 600 mg clindamycin (as clindamycin phosphate, USP) in 0.9% sodium chloride.
- 900 mg/50 mL (18 mg/mL): Each 50 mL single-dose GALAXY container contains 900 mg clindamycin (as clindamycin phosphate, USP) in 0.9% sodium chloride.
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Clostridium difficile Associated Diarrhea
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Clindamycin in 0.9% Sodium Chloride Injection, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated [see Boxed Warning].
5.2 Anaphylactic and Severe Hypersensitivity Reactions
Anaphylactic shock and anaphylactic reactions have been reported [see Adverse Reactions (6)]. Severe hypersensitivity reactions, including severe skin reactions such as toxic epidermal necrolysis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS), and Stevens-Johnson syndrome (SJS), some with fatal outcome, have been reported [see Adverse Reactions (6)].
In case of such an anaphylactic or severe hypersensitivity reaction, discontinue treatment permanently and institute appropriate therapy.
A careful inquiry should be made concerning previous sensitivities to drugs and other allergens.
5.3 Diarrhea in Elderly Patients with Associated Severe Illness
Elderly patients with associated severe illness may have a greater risk of developing adverse reactions from diarrhea. When clindamycin is indicated in these patients, they should be carefully monitored for change in bowel frequency.
5.4 Use in Patients with Gastrointestinal Disease
Clindamycin in 0.9% Sodium Chloride Injection products should be avoided in individuals with a history of gastrointestinal disease, particularly colitis.
5.5 Use in Atopic Individuals
Clindamycin in 0.9% Sodium Chloride Injection should be avoided in atopic individuals.
5.6 Laboratory Tests: Monitoring to Assess Safety
During prolonged therapy periodic liver and kidney function tests and blood counts should be performed.
Clindamycin dosage modification may not be necessary in patients with renal disease. In patients with moderate to severe liver disease, prolongation of clindamycin half-life has been found. However, it was postulated from studies that when given every eight hours, accumulation should rarely occur. Therefore, dosage modification in patients with liver disease may not be necessary. However, periodic liver enzyme determinations should be made when treating patients with severe liver disease.
5.7 Overgrowth of Nonsusceptible Organisms
The use of Clindamycin in 0.9% Sodium Chloride Injection may result in overgrowth of nonsusceptible organisms-particularly yeasts. If such infections occur, appropriate measures should be taken as indicated by the clinical situation.
5.8 Development of Drug-Resistant Bacteria
Prescribing Clindamycin in 0.9% Sodium Chloride Injection in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
-
6 ADVERSE REACTIONS
The following serious adverse reactions to clindamycin are described below and elsewhere in the labeling:
- Clostridium difficile Associated Diarrhea [see Warnings and Precautions (5.1)]
- Anaphylactic and Severe Hypersensitivity Reactions [see Warnings and Precautions (5.2)]
The following adverse reactions associated with the use of clindamycin were identified in clinical trials or postmarketing reports. Because these reactions were reported voluntarily from a population of uncertain size, it is not always possible to estimate their frequency reliably, or to establish a causal relationship to drug exposure.
Infections and Infestations
Clostridium difficile colitis
Gastrointestinal
Antibacterial-associated colitis [see Warnings and Precautions (5.1)], pseudomembranous colitis, abdominal pain, nausea, and vomiting. The onset of pseudomembranous colitis symptoms may occur during or after antibacterial treatment [see Warnings and Precautions (5.1)]. An unpleasant or metallic taste has been reported after intravenous administration of the higher doses of clindamycin phosphate.
Hypersensitivity Reactions
Maculopapular rash and urticaria have been observed during drug therapy. Generalized mild to moderate morbilliform-like skin rashes are the most frequently reported of all adverse reactions.
Severe skin reactions such as Toxic Epidermal Necrolysis, some with fatal outcome, have been reported [see Warnings and Precautions (5.2)]. Cases of Acute Generalized Exanthematous Pustulosis (AGEP), erythema multiforme, some resembling Stevens-Johnson syndrome, have been associated with clindamycin. Anaphylactic shock, anaphylactic reaction and hypersensitivity have also been reported [see Warnings and Precautions (5.2)].
Skin and Mucous Membranes
Pruritus, vaginitis, angioedema and rare instances of exfoliative dermatitis have been reported [see Warnings and Precautions (5.2)].
Liver
Jaundice and abnormalities in liver function tests have been observed during clindamycin therapy.
Renal
Renal dysfunction as evidenced by azotemia, oliguria, and/or proteinuria has been observed.
Hematopoietic
Transient neutropenia (leukopenia) and eosinophilia have been reported. Reports of agranulocytosis and thrombocytopenia have been made.
Immune System
Drug reaction with eosinophilia and systemic symptoms (DRESS) cases have been reported.
Local Reactions
Thrombophlebitis has been reported after intravenous infusion. Avoid prolonged use of indwelling intravenous catheters.
Musculoskeletal
Polyarthritis cases have been reported.
Cardiovascular
Cardiopulmonary arrest and hypotension have been reported following too rapid intravenous administration [see Dosage and Administration (2)].
-
7 DRUG INTERACTIONS
7.1 Neuromuscular Blocking Agents
Clindamycin has been shown to have neuromuscular blocking properties that may enhance the action of other neuromuscular blocking agents. Therefore, it should be avoided in patients receiving such agents.
7.2 Inhibitors of CYP3A4 and CYP3A5
Inhibitors of CYP3A4 and/or CYP3A5 may increase plasma concentrations of clindamycin [see Clinical Pharmacology (12.3)]. Monitor for adverse reactions when strong CYP3A4 and/or CYP3A5 inhibitors are coadministered with clindamycin.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
In limited published clinical trials with pregnant women, the systemic administration of clindamycin during the second and third trimesters, has not been associated with an increased frequency of major birth defects.
The limited published data on use of clindamycin in pregnant women with exposure during the first trimester are insufficient to inform a drug-associated risk of pregnancy-related adverse outcomes [see Data]. In animal reproduction studies, no evidence of any adverse developmental outcomes was observed when oral or subcutaneous doses of clindamycin were administered to pregnant rats and mice during organogenesis at doses half to twice the highest clinically relevant dose based on body surface area comparison [see Data]. The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Human Data
In limited published trials in pregnant women administered clindamycin during the first trimester of pregnancy, there was no difference in the rate of major birth defects reported among in utero exposed infants compared to unexposed infants. From these observational data, it is not possible to draw any conclusions on the rate of specific major birth defects associated with clindamycin. These data cannot definitely establish or exclude any clindamycin-associated risk during pregnancy.
Animal Data
Reproduction studies performed during organogenesis (gestational days 6-15) in pregnant rats and mice that were administered oral doses of clindamycin up to 600 mg/kg/day (twice or equivalent to the highest recommended adult human dose based on a body surface area comparison, respectively) or subcutaneous doses of clindamycin up to 250 mg/kg/day (equivalent to or half the highest recommended adult human dose based on a body surface area comparison, respectively) revealed no evidence of teratogenicity.
8.2 Lactation
Risk Summary
Clindamycin has been reported to appear in breast milk in the range of 0.7 to 3.8 mcg/mL at dosages of 150 mg orally to 600 mg intravenously. Clindamycin has the potential to cause adverse effects on the breastfed infant’s gastrointestinal flora.
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for clindamycin and any potential adverse effects on the breast-fed child from clindamycin or from the underlying maternal condition.
Clinical Considerations
If oral or intravenous clindamycin is required by a nursing mother, it is not a reason to discontinue breastfeeding, but an alternate drug may be preferred. Monitor the infant for possible adverse effects on the gastrointestinal flora, such as diarrhea, candidiasis (thrush, diaper rash) or blood in the stool indicating possible antibacterial-associated colitis.
8.4 Pediatric Use
When Clindamycin in 0.9% Sodium Chloride Injection is administered to the pediatric population (birth to 16 years) appropriate dosing and monitoring of organ system functions is desirable.
The potential for the toxic effect in the pediatric population from chemicals that may leach from the single dose premixed intravenous preparation in plastic has not been evaluated.
8.5 Geriatric Use
Clinical studies of clindamycin did not include sufficient numbers of patients age 65 and over to determine whether they respond differently from younger patients. However, other reported clinical experience indicates that antibacterial-associated colitis and diarrhea (due to Clostridium difficile) seen in association with most antibacterial drugs occur more frequently in the elderly (>60 years) and may be more severe. These patients should be carefully monitored for the development of diarrhea.
Pharmacokinetic studies with clindamycin have shown no clinically important differences between young and elderly subjects with normal hepatic function and normal (age-adjusted) renal function after oral or intravenous administration [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Significant mortality was observed in mice at an intravenous dose of 855 mg/kg (~3 times the human dose based on body surface area) and in rats at an oral or subcutaneous dose of approximately 2618 mg/kg (~18 times the human dose based on body surface area). In the mice, convulsions and depression were observed and in rats depression was observed prior to death.
Hemodialysis and peritoneal dialysis are not effective in removing clindamycin from the serum.
-
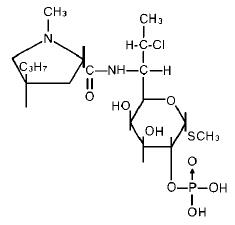
11 DESCRIPTION
Clindamycin is a lincosamide antibacterial and a semisynthetic drug produced by a 7(S) chloro substitution of the 7(R) hydroxyl group of the parent compound lincomycin.
The chemical name of clindamycin phosphate is L-threo-α-D-galacto-Octopyranoside, methyl-7-chloro-6,7,8-trideoxy-6-[[(1-methyl-4-propyl-2-pyrrolidinyl)carbonyl]amino]‑1-thio-, 2-(dihydrogen phosphate), (2S-trans)-.
The molecular formula is C18H34CIN2O8PS and the molecular weight is 504.96.
The structural formula is represented below:
Clindamycin in 0.9% Sodium Chloride Injection is a clear, cloroless and sterile solution for intravenous infusion. Each 50 mL Clindamycin in 0.9% Sodium Chloride Injection, 300 mg/50 mL, 600 mg/50 mL, and 900 mg/50 mL, contains 300 mg, 600 mg or 900 mg clindamycin, respectively (as clindamycin phosphate, USP), 450 mg of sodium chloride USP, 2 mg of edetate disodium dihydrate USP, and water for Injection USP. The pH is adjusted with sodium hydroxide and /or hydrochloric acid. Clindamycin in 0.9% Sodium Chloride Injection is filled in GALAXY plastic container fabricated from a specially designed multilayer plastic, PL 2501. Solutions in contact with the plastic container can leach out certain of its chemical components in very small amounts within the expiration period. The suitability of the plastic has been confirmed in tests in animals according to the USP biological tests for plastic containers, as well as by tissue culture toxicity studies.
-
12 CLINICAL PHARMACOLOGY
12.3 Pharmacokinetics
Biologically inactive clindamycin phosphate is converted to active clindamycin. By the end of short-term intravenous infusion, peak serum concentrations of active clindamycin are reached.
After intramuscular injection of clindamycin phosphate, peak concentrations of active clindamycin are reached within 3 hours in adults and 1 hour in pediatric patients. Serum concentration-time curves may be constructed from intravenous peak serum concentrations as given in Table 2 by application of elimination half-lives described in the elimination section below.
Serum concentrations of clindamycin can be maintained above the in vitro minimum inhibitory concentrations for most indicated organisms by administration of clindamycin phosphate every 8 to 12 hours in adults and every 6 to 8 hours in pediatric patients, or by continuous intravenous infusion. An equilibrium state is reached by the third dose.
Serum assays for active clindamycin require an inhibitor to prevent in vitro hydrolysis of clindamycin phosphate.
Table 2. Average Peak and Trough Serum Concentrations of Active Clindamycin After Dosing with Clindamycin Phosphate - * Data in this group from patients being treated for infection.
Dosage Regimen
Peak
mcg/mL
Trough
mcg/mL
Healthy Adult Males
(Post equilibrium)
- 600 mg IV in 30 min q6h
10.9
2.0
- 600 mg IV in 30 min q8h
10.8
1.1
- 900 mg IV in 30 min q8h
14.1
1.7
Pediatric Patients (first dose)*
- 5–7 mg/kg IV in 1 hour
10
Distribution
No significant concentrations of clindamycin are attained in the cerebrospinal fluid even in the presence of inflamed meninges.
Elimination
Clindamycin is metabolized predominantly by CYP3A4, and to a lesser extent by CYP3A5, to the major metabolite clindamycin sulfoxide and minor metabolite N-desmethylclindamycin. Biologically inactive clindamycin phosphate disappears from the serum with 6 minutes of the average elimination half-life; however, the average serum elimination half-life of active clindamycin is about 3 hours in adults and 2.5 hours in pediatric patients.
Specific Populations
Patients with Renal/Hepatic Impairment
The elimination half-life of clindamycin is increased slightly in patients with markedly reduced renal or hepatic function. Hemodialysis and peritoneal dialysis are not effective in removing clindamycin from the serum. Dosage schedules need not be modified in the presence of mild or moderate renal or hepatic disease.
Geriatric Patients
Pharmacokinetic studies in elderly volunteers (61-79 years) and younger adults (18-39 years) indicate that age alone does not alter clindamycin pharmacokinetics (clearance, elimination half-life, volume of distribution, and area under the serum concentration-time curve) after intravenous administration of clindamycin phosphate. After oral administration of clindamycin hydrochloride, the average elimination half-life is increased to approximately 4.0 hours (range 3.4-5.1 h) in the elderly, compared to 3.2 hours (range 2.1-4.2 h) in younger adults. The extent of absorption, however, is not different between age groups and no dosage alteration is necessary for the elderly with normal hepatic function and normal (age-adjusted) renal function1.
Drug Interaction Studies
In vitro studies indicate that clindamycin does not inhibit CYP1A2, CYP2C9, CYP2C19, CYP2E1 or CYP2D6 and only moderately inhibits CYP3A4.
12.4 Microbiology
Mechanism of Action
Clindamycin inhibits bacterial protein synthesis by binding to the 23S RNA of the 50S subunit of the ribosome. Clindamycin is bacteriostatic.
Resistance
Resistance to clindamycin is most often caused by modification of specific bases of the 23S ribosomal RNA. Cross-resistance between clindamycin and lincomycin is complete. Because the binding sites for these antibacterial drugs overlap, cross-resistance is sometimes observed among lincosamides, macrolides and streptogramin B. Macrolide-inducible resistance to clindamycin occurs in some isolates of macrolide-resistant bacteria. Macrolide-resistant isolates of staphylococci and beta-hemolytic streptococci should be screened for induction of clindamycin resistance using the D-zone test.
Antimicrobial Activity
Clindamycin has been shown to be active against most of the isolates of the following microorganisms, both in vitro and in clinical infections [see Indications and Usage (1)].
-
Aerobic bacteria
- Gram-positive bacteria
-
Staphylococcus aureus (methicillin-susceptible strains)
Streptococcus pneumoniae (penicillin-susceptible strains)
Streptococcus pyogenes - Anaerobic bacteria
- Gram-positive bacteria
-
Clostridium perfringens
Peptostreptococcus anaerobius - Gram-negative bacteria
-
Fusobacterium necrophorum
Fusobacterium nucleatum
Prevotella melaninogenica
The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following bacteria exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for clindamycin against isolates of similar genus or organism group. However, the efficacy of clindamycin in treating clinical infections caused by these bacteria has not been established in adequate and well-controlled clinical trials.
- Aerobic bacteria
- Gram-positive bacteria
-
Staphylococcus epidermidis (methicillin-susceptible strains)
Streptococcus agalactiae
Streptococcus anginosus
Streptococcus mitis
Streptococcus oralis - Anaerobic bacteria
- Gram-positive bacteria
-
Actinomyces israelii
Clostridium clostridioforme
Eggerthella lenta
Finegoldia (Peptostreptococcus) magna
Micromonas (Peptostreptococcus) micros
Propionibacterium acnes - Gram-negative bacteria
-
Prevotella bivia
Prevotella intermedia
Susceptibility Test Methods
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: http://www.fda.gov/STIC.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long term studies in animals have not been performed with clindamycin to evaluate carcinogenic potential. Genotoxicity tests performed included a rat micronucleus test and an Ames Salmonella reversion test. Both tests were negative.
Fertility studies in rats treated orally with up to 300 mg/kg/day (approximately 1.1 times the highest recommended adult human dose based on mg/m2) revealed no effects on fertility or mating ability.
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Clindamycin in 0.9% Sodium Chloride Injection for intravenous infusion. Each 50 mL of Clindamycin in 0.9% Sodium Chloride Injection, 300 mg/50 mL, 600 mg/50 mL, and 900 mg/50 mL, contains 300 mg, 600 mg or 900 mg clindamycin, respectively (as clindamycin phosphate, USP). Clindamycin in 0.9% Sodium Chloride Injection in single-dose GALAXY containers is available as follows:
2G3455
Twenty-four (24)-300 mg/50 mL containers
NDC: 0338-9545-24
2G3456
Twenty-four (24)-600 mg/50 mL containers
NDC: 0338-9549-24
2G3457
Twenty-four (24)-900 mg/50 mL containers
NDC: 0338-9553-24
Exposure of pharmaceutical products, including Clindamycin in 0.9 % Sodium Chloride, to heat should be minimized. It is recommended that GALAXY plastic containers be stored at 20°C to 25°C (68°F to 77°F) excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature]. Avoid temperatures above 30°C.
-
17 PATIENT COUNSELING INFORMATION
- Counsel patients that antibacterial drugs including Clindamycin in 0.9% Sodium Chloride Injection should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Clindamycin in 0.9% Sodium Chloride Injection is prescribed to treat a bacterial infection, inform patient that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Clindamycin in 0.9% Sodium Chloride Injection or other antibacterial drugs in the future.
- Counsel patients that diarrhea is a common problem caused by antibacterial drugs which usually ends when the antibacterial is discontinued. Sometimes after starting treatment with antibacterial drugs, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibacterial drugs. If this occurs, contact physician as soon as possible [see Warnings and Precautions (5.4)].
- Lactation: Advise a woman to monitor the breastfed infant for diarrhea and bloody stools [see Use in Specific Populations (8.2)].
Baxter and Galaxy are registered trademarks of Baxter International Inc.
Baxter Healthcare Corporation
Deerfield, IL 60015 USAMade in USA.
07-19-01-299
-
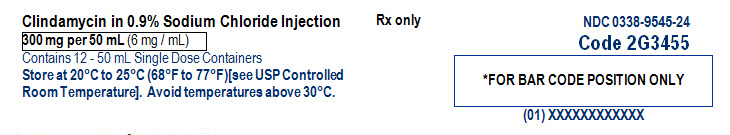
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC: 0338-9545-50
Clindamycin in 0.9% Sodium Chloride Injection300 mg per 50 mL
(6 mg / mL)50 mL Single Dose GALAXY Container
Code 2G3455Discard unused portion
Sterile NonpyrogenicFor Intravenous Infusion Only
Cautions: Do not add supplementary medication. Must not be used in series
connections. Check for minute leaks and solution clarity.Each 50 mL contains: Clindamycin phosphate, USP equivalent to 300 mg
clindamycin, 450 mg sodium chloride, USP, 2 mg edetate disodium dihydrate,
USP, and Water for Injection, USP. pH may have been adjusted with sodium
hydroxide and/or hydrochloric acid.Dosage: See prescribing information.
Rx only
Store at 20°C to 25°C (68°F to 77°F)[see USP Controlled Room Temperature].
Avoid temperatures above 30°C.PL 2501 Plastic
BAXTER Logo
Baxter and Galaxy are registered trademarks of Baxter International Inc.
Baxter Healthcare Corporation, Deerfield, IL 60015 USA
Made in USA07-34-00-0002
GALAXY Container PL 2501 Plastic
BAXTER Logo
Baxter and Galaxy are registered trademarks of Baxter International Inc.
Baxter Healthcare Corporation, Deerfield, IL 60015 USA
Made in USAClindamycin in 0.9% Sodium Chloride Injection
Rx only
NDC: 0338-9545-24300 mg per 50 mL (6 mg / mL)
Code 2G3455Contains 12 - 50 mL Single Dose Containers
Store at 20°C to 25°C (68°F to 77°F) [see USP Controlled
Room Temperature]. Avoid temperatures above 30°C.FOR BAR CODE POSITION ONLY
(01) XXXXXXXXXXXXFor Intravenous Infusion Only
Sterile NonpyrogenicEach 50 mL contains: Clindamycin phosphate, USP equivalent to 300 mg clindamycin, 450 mg sodium
chloride, USP, 2 mg edetate disodium dihydrate, USP, and Water for Injection, USP. pH may have been adjusted with
sodium hydroxide and/or hydrochloric acid.
Dosage: See prescribing information.
Cautions: Do not add supplementary medication. Must not be used in series connections. Check for minute
leaks and solution clarity.Clindamycin in 0.9% Sodium Chloride
Injection 300mg/50mL
Code 2G3455 NDC: 0338-9545-24Store at 20°C to 25°C (68°F to 77°F)
[see USP Controlled Room Temperature].
Avoid temperatures above 30°C.Rx Only
Baxter Logo
Baxter Healthcare Corporation
Deerfield, IL 60015 USALOT NC123456
EXP DD MMM YY
SN 0123456789Clindamycin in 0.9% Sodium Chloride
Injection 300mg/50mL
Code 2G3455 NDC: 0338-9545-24Rx Only
Baxter Logo
Baxter Healthcare Corporation
Deerfield, IL 60015 USAStore at 20°C to 25°C (68°F to 77°F)
[see USP Controlled Room Temperature].
Avoid temperatures above 30°C.LOT NC123456
EXP DD MMM YY
SN 012345678907-06-77-459
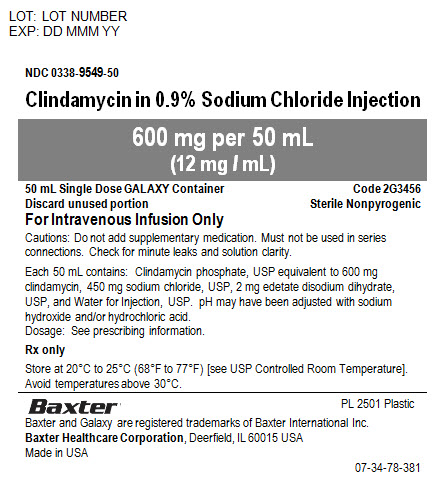
NDC 0338-9549-50
Clindamycin in 0.9% Sodium Chloride Injection600 mg per 50 mL
(12 mg / mL)50 mL Single Dose GALAXY Container
Code 2G3456Discard unused portion
Sterile Nonpyrogenic
For Intravenous Use OnlyCautions: Do not add supplementary medication. Must not be used in series connections. Check for minute leaks and solution clarity.
Each 50 mL contains: Clindamycin phosphate, USP equivalent to 600 mg clindamycin, 450 mg sodium chloride, USP, 2 mg edetate disodium dihydrate, USP, and Water for Injection, USP. pH may have been adjusted with sodium hydroxide and/or hydrochloric acid.
Dosage: See prescribing information.
Rx only
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Avoid temperatures above 30°C.
PL 2501 Plastic
BAXTER Logo
Baxter and Galaxy are registered trademarks of Baxter International Inc.
Baxter Healthcare Corporation, Deerfield, IL 60015 USA
Made in USA07-34-78-381
GALAXY Container
PL 2501 PlasticBAXTER Logo
Baxter and Galaxy are registered trademarks of Baxter International Inc.
Baxter Healthcare Corporation, Deerfield, IL 60015 USA
Made in USA07-04-78-379
Clindamycin in 0.9% Sodium Chloride Injection
Rx only
NDC: 0338-9549-24600 mg per 50 mL (12 mg / mL)
Code 2G3456Contains 12 - 50 mL Single Dose Containers
Store at 20°C to 25°C (68°F to 77°F)[see USP Controlled
Room Temperature]. Avoid temperatures above 30°C.FOR BAR CODE POSITION ONLY
(01) XXXXXXXXXXXXFor Intravenous Infusion Only
Sterile NonpyrogenicEach 50 mL contains: Clindamycin phosphate, USP equivalent to 600 mg clindamycin, 450 mg sodium
chloride, USP, 2 mg edetate disodium dihydrate, USP, and Water for Injection, USP. pH may have been
adjusted with sodium hydroxide and/or hydrochloric acid.Dosage: See prescribing information.
Cautions: Do not add supplementary medication. Must not be used in series connections. Check for minute
leaks and solution clarity.Clindamycin in 0.9% Sodium Chloride
Injection 600mg/50mL
Code 2G3456 NDC: 0338-9549-24Store at 20°C to 25°C (68°F to 77°F)
[see USP Controlled Room Temperature].
Avoid temperatures above 30°C.Rx Only
Baxter Logo
Baxter Healthcare Corporation
Deerfield, IL 60015 USALOT NC123456
EXP DD MMM YY
SN 0123456789Clindamycin in 0.9% Sodium Chloride
Injection 600mg/50mL
Code 2G3456 NDC: 0338-9549-24Rx Only
Baxter Logo
Baxter Healthcare Corporation
Deerfield, IL 60015 USAStore at 20°C to 25°C (68°F to 77°F)
[see USP Controlled Room Temperature].
Avoid temperatures above 30°C.LOT NC123456
EXP DD MMM YY
SN 012345678907-06-77-460
NDC: 0338-9553-50
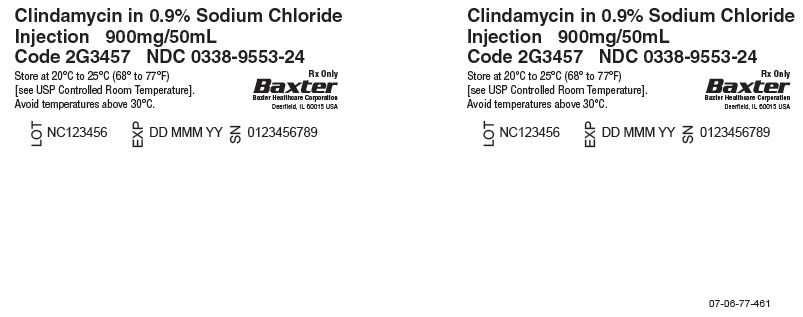
Clindamycin in 0.9% Sodium Chloride Injection900 mg per 50 mL
(18 mg / mL)-
50 mL Single Dose GALAXY Container
Discard unused portion
Code 2G3457
Sterile Nonpyrogenic
For Intravenous Infusion Only
Cautions: Do not add supplementary medication. Must not be used in series
connections. Check for minute leaks and solution clarity.Each 50 mL contains: Clindamycin phosphate, USP equivalent to 900 mg
clindamycin, 450 mg sodium chloride, USP, 2 mg edetate disodium dihydrate,
USP, and Water for Injection, USP. pH may have been adjusted with sodium
hydroxide and/or hydrochloric acid.
Dosage: See prescribing information.Rx only
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Avoid temperatures above 30°C.PL 2501 Plastic
BAXTER Logo
Baxter and Galaxy are registered trademarks of Baxter International Inc.
Baxter Healthcare Corporation, Deerfield, IL 60015 USA
Made in USA07-34-78-382
GALAXY Container
PL 2501 PlasticBAXTER Logo
Baxter and Galaxy are registered trademarks of Baxter International Inc.
Baxter Healthcare Corporation, Deerfield, IL 60015 USA
Made in USA07-04-78-380
Clindamycin in 0.9% Sodium Chloride Injection
Rx only
NDC: 0338-9553-24900 mg per 50 mL (18 mg / mL)
Code 2G3457Contains 12 - 50 mL Single Dose Containers
Store at 20°C to 25°C (68°F to 77°F)[see USP Controlled
Room Temperature]. Avoid temperatures above 30°C.FOR BAR CODE POSITION ONLY
(01) XXXXXXXXXXXXFor Intravenous Infusion Only
Sterile NonpyrogenicEach 50 mL contains: Clindamycin phosphate, USP equivalent to 900 mg clindamycin, 450 mg sodium
chloride, USP, 2 mg edetate disodium dihydrate, USP, and Water for Injection, USP. pH may have been
adjusted with sodium hydroxide and/or hydrochloric acid.Dosage: See prescribing information.
Caution: Do not add supplementary medication. Must not be used in series connections. Check for minute
leaks and solution clarity.Clindamycin in 0.9% Sodium Chloride
Injection 900mg/50mL
Code 2G3457 NDC: 0338-9553-24Store at 20°C to 25°C (68°F to 77°F)
[see USP Controlled Room Temperature].
Avoid temperatures above 30°C.Rx Only
Baxter Logo
Baxter Healthcare Corporation
Deerfield, IL 60015 USALOT NC123456
EXP DD MMM YY
SN 0123456789Clindamycin in 0.9% Sodium Chloride
Injection 900mg/50mL
Code 2G3457 NDC: 0338-9553-24Rx Only
Baxter Logo
Baxter Healthcare Corporation
Deerfield, IL 60015 USAStore at 20°C to 25°C (68°F to 77°F)
[see USP Controlled Room Temperature].
Avoid temperatures above 30°C.LOT NC123456
EXP DD MMM YY
SN 012345678907-06-77-461
-
50 mL Single Dose GALAXY Container
-
INGREDIENTS AND APPEARANCE
CLINDAMYCIN PHOSPHATE
clindamycin phosphate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0338-9545 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLINDAMYCIN PHOSPHATE (UNII: EH6D7113I8) (CLINDAMYCIN - UNII:3U02EL437C) CLINDAMYCIN PHOSPHATE 300 mg in 50 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) 2.00 mg in 50 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 9.00 mg in 50 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-9545-24 24 in 1 CARTON 04/20/2017 1 NDC: 0338-9545-50 50 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208083 04/20/2017 CLINDAMYCIN PHOSPHATE
clindamycin phosphate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0338-9549 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLINDAMYCIN PHOSPHATE (UNII: EH6D7113I8) (CLINDAMYCIN - UNII:3U02EL437C) CLINDAMYCIN PHOSPHATE 600 mg in 50 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) 2.00 mg in 50 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 9.00 mg in 50 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-9549-24 24 in 1 CARTON 04/20/2017 1 NDC: 0338-9549-50 50 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208083 04/20/2017 CLINDAMYCIN PHOSPHATE
clindamycin phosphate injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0338-9553 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CLINDAMYCIN PHOSPHATE (UNII: EH6D7113I8) (CLINDAMYCIN - UNII:3U02EL437C) CLINDAMYCIN PHOSPHATE 900 mg in 50 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) 2.00 mg in 50 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) 9.00 mg in 50 mL SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0338-9553-24 24 in 1 CARTON 04/20/2017 1 NDC: 0338-9553-50 50 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208083 04/20/2017 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter Healthcare Corporation 194684502 ANALYSIS(0338-9545, 0338-9549, 0338-9553) , LABEL(0338-9545, 0338-9549, 0338-9553) , MANUFACTURE(0338-9545, 0338-9549, 0338-9553) , PACK(0338-9545, 0338-9549, 0338-9553) , STERILIZE(0338-9545, 0338-9549, 0338-9553)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.