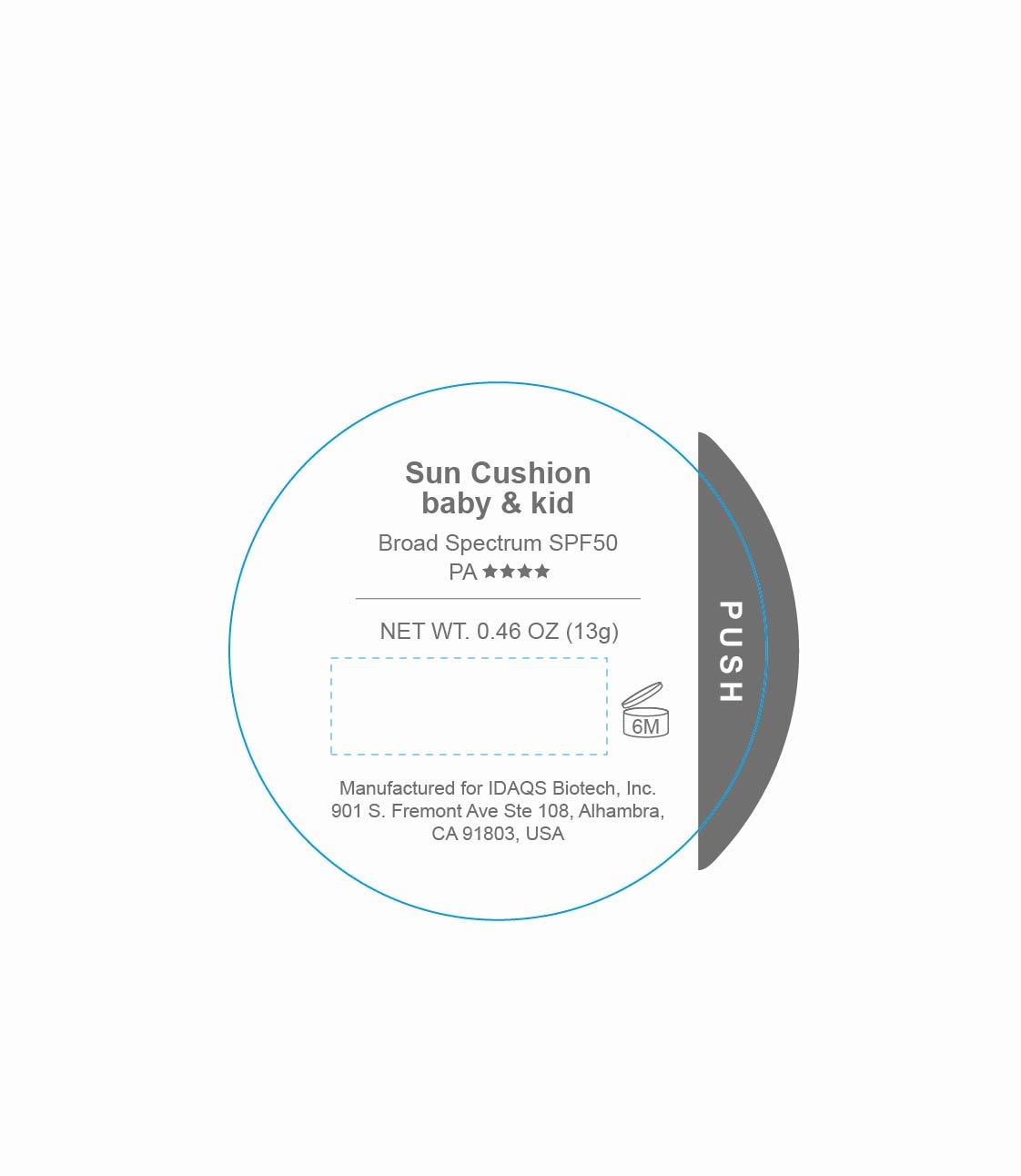
UNID Sun Cushion baby and kid SPF50
UNID Sun Cushion baby and kid by
Drug Labeling and Warnings
UNID Sun Cushion baby and kid by is a Otc medication manufactured, distributed, or labeled by Sage Pharmaceuticals Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
UNID SUN CUSHION BABY AND KID- titanium dioxide, zinc oxide lotion
Sage Pharmaceuticals Inc
----------
UNID Sun Cushion baby and kid SPF50
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
- Apply liberally 15 minutes before sun exposure
- Reapply at least every 2 hours
- Use a water-resistant sunscreen if swimming or sweating
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging.
- To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m.–2 p.m.
- Wear long-sleeve shirts, pants, hats, and sunglasses
- Children under 6 months of age: ask a doctor.
Inactive Ingredients
Water, Coco-Caprylate/Caprate, Caprylic/Capric Triglyceride, Octyldodecanol, Polyglyceryl-3 Diisostearate, Saccharomyces/Rice Ferment Filtrate, Pentylene Glycol, Aluminum Hydroxide, Stearic Acid, Sodium Chloride, Tocopheryl Acetate, Triethoxycaprylylsilane, Propanediol, Laminaria Ochroleuca Extract, Sodium Hyaluronate, Butylene Glycol, Hydrolyzed Sodium Hyaluronate.
| UNID SUN CUSHION BABY AND KID
titanium dioxide, zinc oxide lotion |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Sage Pharmaceuticals Inc (656245476) |
| Registrant - Sage Pharmaceuticals Inc (656245476) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sage Pharmaceuticals Inc | 656245476 | manufacture(83119-001) | |
Revised: 12/2025
Document Id: 4591a759-0cba-7a0c-e063-6394a90a9ad2
Set id: 4531a2d7-4580-49f7-e063-6294a90a19fa
Version: 3
Effective Time: 20251209