TORK FOAM ANTIBACTERIAL- benzalkonium chloride soap
Tork Foam Antibacterial by
Drug Labeling and Warnings
Tork Foam Antibacterial by is a Otc medication manufactured, distributed, or labeled by Essity Professional Hygiene North America LLC, CYAN Labs S.A. de C.V.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Inactive ingredients
- Questions or Comments?
-
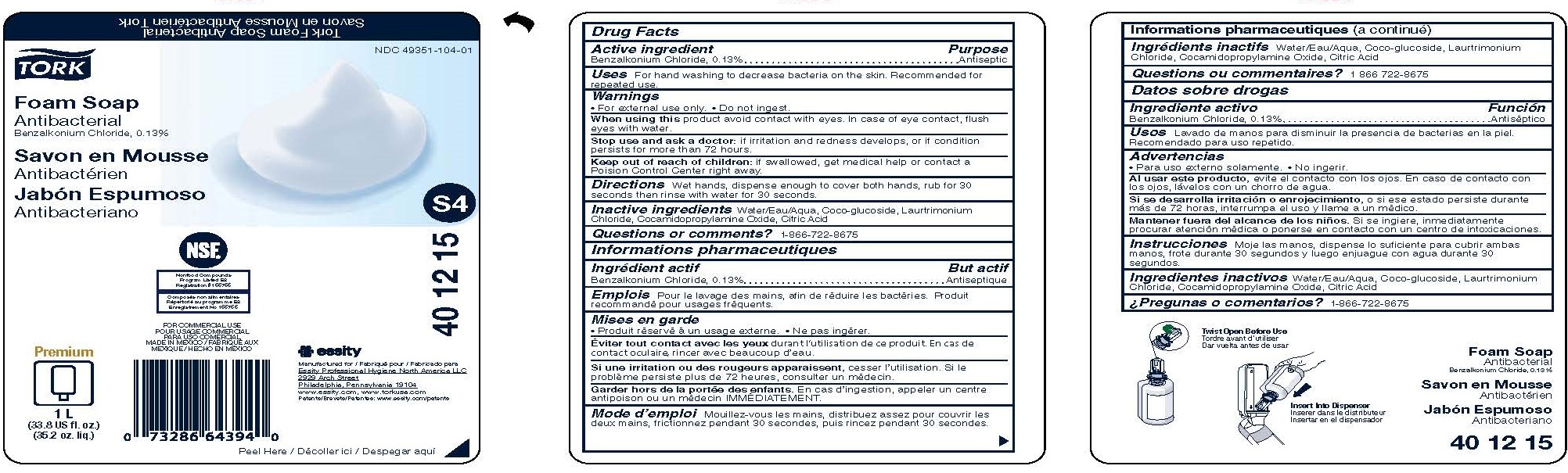
PRINCIPAL DISPLAY PANEL
TORK®
Foam Soap
Antibacterial
NDC: 49351-104-01
Manufactured for Essity Professional Hygiene North America, LLC
2929 Arch Street
Philadelphia, PA 19104
www.torkusa.com
For Commercial use
Made in Mexico
Premium
1 L
(33.8 US fl. oz.)
(35.2 oz. liq.)

-
INGREDIENTS AND APPEARANCE
TORK FOAM ANTIBACTERIAL
benzalkonium chloride soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 49351-104 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL Inactive Ingredients Ingredient Name Strength COCO GLUCOSIDE (UNII: ICS790225B) LAURTRIMONIUM CHLORIDE (UNII: A81MSI0FIC) WATER (UNII: 059QF0KO0R) COCAMIDOPROPYLAMINE OXIDE (UNII: M4SL82J7HK) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49351-104-01 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/15/2017 2 NDC: 49351-104-02 24 in 1 BOX 03/15/2017 2 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 03/15/2017 Labeler - Essity Professional Hygiene North America LLC (005694349) Registrant - CYAN Labs S.A. de C.V. (812754130) Establishment Name Address ID/FEI Business Operations CYAN Labs S.A. de C.V. 812754130 manufacture(49351-104) , label(49351-104) , pack(49351-104)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.