LODINE by Dongguan Tianli Material Technology Co., Ltd.
LODINE by
Drug Labeling and Warnings
LODINE by is a Otc medication manufactured, distributed, or labeled by Dongguan Tianli Material Technology Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
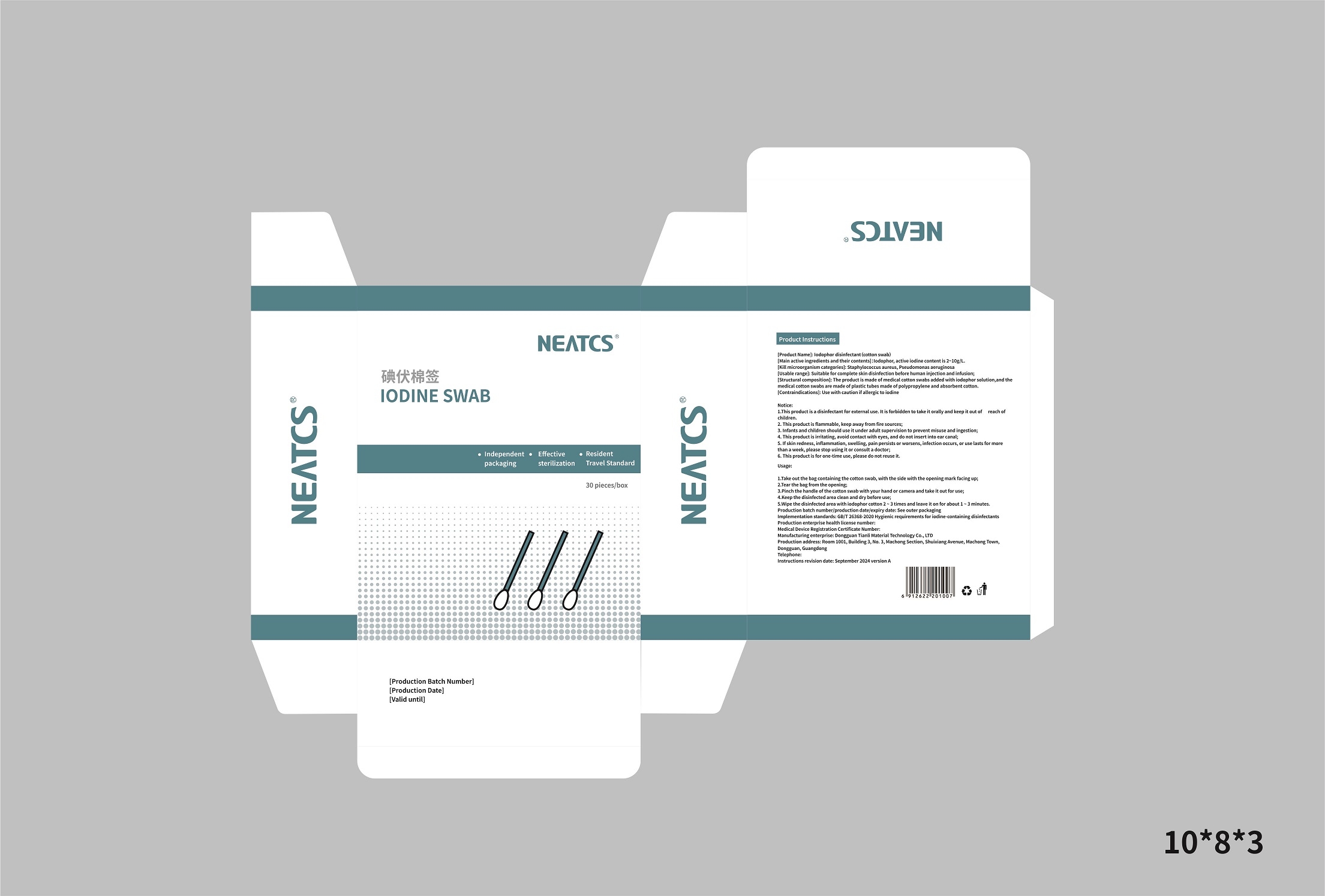
LODINE- povidone-iodine swab
Dongguan Tianli Material Technology Co., Ltd.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Notice
1.This product is a disinfectant for external use. it is forbidden to take it orally and keep it out ofreach of children.
2.This product is flammable, keep away from fire sources.
3. infants and children should use it under adult supervision to prevent misuse and ingestion.
4. This product is irritating, avoid contact with eyes, and do not insert into ear canal.
5. lf skin redness, inflammation, swelling, pain persists or worsens, infection occurs, or use lasts formore than a week, please stop using it or consult a doctor
6. This product is for one-time use, please do not reuse it.
Notice
5. lf skin redness, inflammation, swelling, pain persists or worsens, infection occurs, or use lasts for more than a week,please stop using it or consult a doctor
Notice:
1.This product is a disinfectant for external use. lt is forbidden to take it orally and keep it out of
reach of children.
Usage
1.Take out the bag containing,the cotton swab, with the side with the opening mark facing upg
2.Tear the bag from the openings
3.Pinch the handle of the cotton swab with your hand or camera and take it out for use;
4.Keep the disinfected area clean and dry before use;
5.wipe the disinfected area with iodophor cotton 2 ~ 3 times and leave it on for about 1 ~3 minutes.
| LODINE
povidone-iodine swab |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Dongguan Tianli Material Technology Co., Ltd. (453433114) |
| Registrant - Dongguan Tianli Material Technology Co., Ltd. (453433114) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Dongguan Tianli Material Technology Co., Ltd. | 453433114 | manufacture(85175-002) | |
Trademark Results [LODINE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
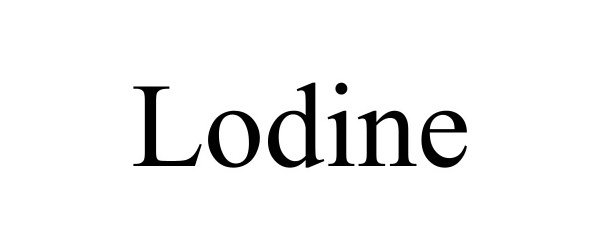 LODINE 98499210 not registered Live/Pending |
Galt Pharmaceuticals, LLC 2024-04-14 |
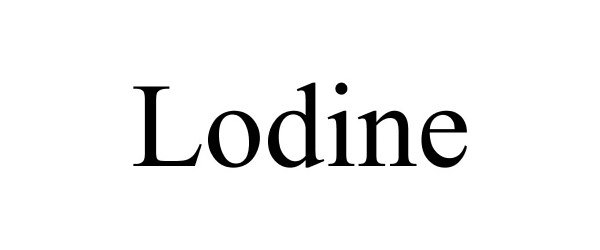 LODINE 87137124 5129189 Live/Registered |
Cutis Health, LLC 2016-08-12 |
 LODINE 78359563 3023014 Dead/Cancelled |
SHIONOGI INC. 2004-01-29 |
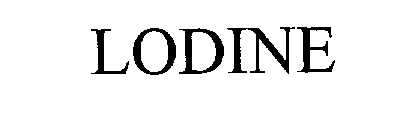 LODINE 76232699 2512709 Dead/Cancelled |
American Home Products Corporation 2001-03-28 |
 LODINE 74175962 1736010 Dead/Cancelled |
AMERICAN HOME PRODUCTS CORPORATION 1991-06-14 |
 LODINE 73807418 1581039 Dead/Cancelled |
AMERICAN HOME PRODUCTS CORPORATION 1989-06-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.