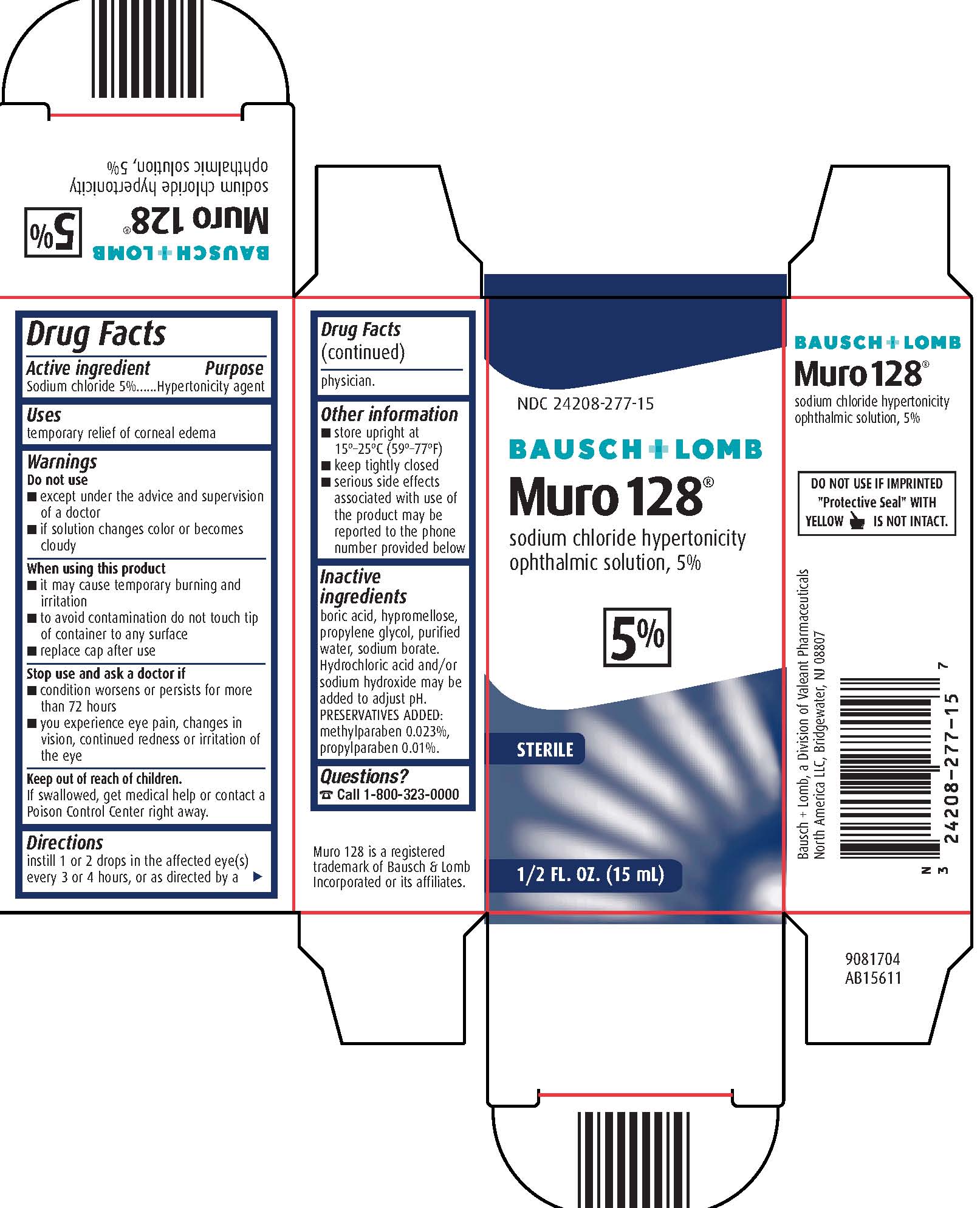
MURO 128- sodium chloride solution
Muro 128 by
Drug Labeling and Warnings
Muro 128 by is a Otc medication manufactured, distributed, or labeled by Bausch & Lomb Incorporated. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- Active ingredients
- Purpose
- Uses
-
Warnings
Do not use
- except under the advice and supervision of a doctor
- if solution changes color or becomes cloudy
When using this product
- it may cause temporary burning and irritation
- to avoid contamination do not touch tip of container to any surface
- replace cap after use
Stop use and ask a doctor if
- condition worsens or persists for more than 72 hours
- you experience eye pain, changes in vision, continued redness or irritation of the eye
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Questions?
-
Package/Label Principal Display Panel – Muro 128 15 mL
NDC: 24208-277-15
BAUSCH + LOMB
Muro 128®
sodium chloride hypertonicity
ophthalmic solution, 5%
5%
STERILE
1/2 FL. OZ. (15 mL)
-
INGREDIENTS AND APPEARANCE
MURO 128
sodium chloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 24208-277 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength BORIC ACID (UNII: R57ZHV85D4) HYPROMELLOSE 2208 (100 MPA.S) (UNII: B1QE5P712K) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BORATE (UNII: 91MBZ8H3QO) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 24208-277-15 1 in 1 CARTON 01/01/2011 1 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 24208-277-30 1 in 1 CARTON 01/01/2011 2 30 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part349 01/01/2011 Labeler - Bausch & Lomb Incorporated (196603781) Establishment Name Address ID/FEI Business Operations Bausch & Lomb Incorporated 079587625 MANUFACTURE(24208-277)
Trademark Results [Muro 128]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MURO 128 78905949 3241901 Live/Registered |
BAUSCH & LOMB INCORPORATED 2006-06-12 |
 MURO 128 73278983 1251448 Dead/Cancelled |
Muro Pharmaceutical, Inc. 1980-09-22 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.