PRASCEND- pergolide tablet
Prascend by
Drug Labeling and Warnings
Prascend by is a Animal medication manufactured, distributed, or labeled by Boehringer Ingelheim Animal Health USA Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Caution
-
Description
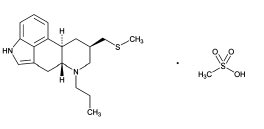
Prascend Tablets are rectangular light red colored, half-scored tablets containing 1 mg pergolide, as pergolide mesylate. Pergolide mesylate is a synthetic ergot derivative and is a potent dopamine receptor agonist. The chemical name of pergolide mesylate is 8β-[(Methylthio) methyl]-6-propylergoline monomethanesulfonate.
The chemical structure is:
- Indication
-
Dosage and Administration
Administer orally at a starting dose of 2 mcg/kg once daily. Dosage may be adjusted to effect, not to exceed 4 mcg/kg daily. It has been reported that pergolide tablets may cause eye irritation, an irritating smell, or headache when Prascend Tablets are split or crushed. Prascend Tablets should not be crushed due to the potential for increased human exposure and care should be taken to minimize exposure when splitting tablets.
The tablets are scored and the calculated dosage should be provided to the nearest one-half tablet increment (see Table 1).
Table 1 Dosing Table Dosage
Dosage
Body weight
2 mcg/kg
4 mcg/kg
136 - 340 kg
(300 - 749 lb)
0.5 tablet
1 tablet
341 - 567 kg
750 - 1,249 lb)
1 tablet
2 tablets
568 - 795 kg
(1,250 - 1,749 lb)
1.5 tablets
3 tablets
796 - 1,022 kg
(1,750 - 2,249 lb)
2 tablets
4 tablets
Dosing should be titrated according to individual response to therapy to achieve the lowest effective dose. Dose titration is based on improvement in clinical signs associated with Pituitary Pars Intermedia Dysfunction (PPID) and/or improvement or normalization of endocrine tests (for example, dexamethasone suppression test or endogenous ACTH test). If signs of dose intolerance develop, the dose should be decreased by half for 3 to 5 days and then titrated back up in 2 mcg/kg increments every 2 weeks until the desired effect is achieved.
- Contraindications
- Warnings
-
Human Warnings
Not for use in humans. Keep this and all medications out of the reach of children. Prascend should not be administered by persons who have had adverse reactions to ergotamine or other ergot derivatives. Pregnant or lactating women should wear gloves when administering this product. It has been reported that pergolide tablets may cause eye irritation, an irritating smell, or headache when Prascend Tablets are split or crushed. Prascend Tablets should not be crushed due to the potential for increased human exposure and care should be taken to minimize exposure when splitting tablets. Consult a physician in case of accidental ingestion by humans.
-
Precautions
Treatment with Prascend may cause inappetance.
The use of Prascend in breeding, pregnant, or lactating horses has not been evaluated. The effects of pergolide mesylate on breeding, pregnant, or lactating horses are not known; however, the pharmacologic action of pergolide mesylate suggests that it may interfere with reproductive functions such as lactation.
Prascend is approximately 90% associated with plasma proteins. Use caution if administering Prascend with other drugs that affect protein binding. Dopamine antagonists, such as neuroleptics (phenothiazines, domperidone) or metoclopramide, ordinarily should not be administered concurrently with Prascend (a dopamine agonist) since these agents may diminish the effectiveness of Prascend.
-
Adverse Reactions
A total of 122 horses treated with Prascend Tablets for six months were included in a field study safety analysis.
Table 2 Summary of the most common adverse reactions(N=122) Clinical Sign
# Cases
Cases (%)
Decreased appetite
40
32.8
Lameness
22
18.0
Diarrhea/Loose stool
12
9.8
Colic
12
9.8
Lethargy
12
9.8
Abnormal Weight Loss
11
9.0
Laminitis*
10
8.2
Heart murmur
10
8.2
Death
8
6.6
Tooth disorder
8
6.6
Skin abscess
7
5.7
Musculoskeletal pain
6
4.9
Behavior change
6
4.9
*Three new cases and 7 pre-existing, recurring cases
Inappetance or decreased appetite occurred at one or more meals in 40 of 122 horses treated with Prascend. At the baseline evaluation 1.6% of owners reported a history of inappetance or decreased appetite as compared to the 32.8% of horses that experienced inappetance or decreased appetite during the study. Most cases of inappetance were transient and occurred during the first month of treatment; however, some horses experienced sporadic inappetance throughout the study. Two horses required a temporary reduction in dose due to inappetance during the first month of the study. Both horses returned to their original dose within 30 days.
Weight loss occurred in more than half of the horses in this study; however, weight loss that was considered abnormal was only reported in 11 horses.
Lethargy was reported in 9.8% of horses during the study, and was not reported in any horses at the baseline evaluation.
Behavioral changes were noted in 6 horses including aggression, kicking, agitation, nervous behavior and increased activity. One horse required a temporary reduction in dose due to energetic behavior during the first month of the study.
Eight horses died or were euthanized during the study due to worsening of pre-existing conditions (laminitis, dental disease, septic tenosynovitis) or colic (strangulating lipomas, large colon volvulus).
One mare was inadvertently enrolled in the study while pregnant and experienced dystocia resulting in the death of the foal.
To report suspected adverse reactions, to obtain a Material Safety Data Sheet (MSDS), or for technical assistance, call 1-866-638-2226.
-
Clinical Pharmacology
Pergolide mesylate is a synthetic ergot derivative and is a potent dopamine receptor agonist. As with other dopamine agonists, pergolide inhibits the release of prolactin which suggests that it may interfere with lactation. In horses with PPID, pergolide is believed to exert its therapeutic effect by stimulating dopamine receptors, and has been shown to decrease the plasma levels of adrenocorticotropic hormone (ACTH), melanocyte stimulating hormone (MSH), and other pro-opiomelanocortin peptides.1
Pharmacokinetic information in the horse is based on a study using single oral doses of 10 mcg/kg in six healthy mares between 3 and 17 years of age.2 Pergolide was rapidly absorbed; the mean maximum concentration (Cmax) was 4.05±2.02 ng/mL with the median time to maximum concentration (Tmax) being 0.415 hours.
The area under the curve (AUC) was 14.08±7.46 hr∙ng/mL. The mean half life (T1/2) was 5.86±3.42 hours; the mean apparent oral clearance (CL/F) was 1204 mL/kg/hr; and the mean apparent volume of distribution (V/F) was 3082±1354 mL/kg.
-
Effectiveness
An open-label, historical control, field study evaluated the effectiveness of Prascend for the control of clinical signs of PPID. A total of 122 horses with PPID were enrolled in the study, 113 of which were included in effectiveness evaluations. The success of each horse was based on results of endocrinology testing (dexamethasone suppression test or endogenous ACTH test) and/or improvement in clinical signs related to PPID (hirsutism, hyperhidrosis, polyuria/polydypsia, abnormal fat distribution, and/or muscle wasting) on the Day 180 evaluation. Based on endocrine testing and investigators' clinical assessment scores, 86 (76.1%) of the 113 evaluable cases were treatment successes.
Table 3 Proportion of Treatment Successes on Day 180 Percent Success
Lower bound: one-sided 95% confidence interval
76.1% (86/113)
68.6%
Enrolled horses were diagnosed with PPID based on the presence of hirsutism and an abnormal pre-study endocrine test result. All horses were treated with 2 mcg/kg Prascend (to the nearest one-half tablet) orally once daily for the first three months. If the endocrine test result on Day 90 was normal or adequately improved, the horse continued on the same dose through Day 180. If the endocrine test result on Day 90 was abnormal, the dose increased to 4 mcg/kg given once daily through Day 180. Forty-seven (41.6%) of the 113 horses included in the effectiveness database required a dose increase at Day 90.
Improvement was noted in scores for all clinical sign categories and in mean results for endocrine tests.
Table 4 Percent of Animals with Improvement in Clinical Signs Relative to Baseline Scores Clinical Sign
Day 90±7 (%)
Day 180±7 (%)
Hirsutism
32.7%
89.2%
Hyperhidrosis
27.4%
42.3%
Polyuria/polydypsia
31.0%
34.2%
Abnormal fat distribution
21.2%
33.3%
Muscle wasting
36.3%
46.0%
Table 5 Endocrine test results (mean values) Test
# Animals
Baseline
Day 90
Day 180
ACTH
(pg/mL)
20
73.53
51.12
45.08
DST**
(mcg/dL)
93
3.12
1.39
1.47
*Dexamethasone suppression test: Post dexamethasone cortisol concentration
-
Animal Safety
In a six month target animal safety study healthy adult horses received Prascend administered orally, once daily, at doses of either 0 mcg/kg, 4 mcg/kg, 6 mcg/kg, or 8 mcg/kg (0X, 1X, 1.5X, or 2X the maximum recommended dose). There were eight healthy horses (four males and four females) in each treatment group. Doses were prepared by dissolving tablets in approximately 10 mL of a 50% sugar water solution.
Prascend treated groups had lower mean heart rates and higher mean temperatures than the control group. Horses in all treatment groups had minimum heart rates within the normal range and maximum temperatures below 101.5°F. One 1.5X horse experienced a mild episode of spasmodic colic on Day 3 that resolved after treatment with flunixin meglumine.
Mean red blood cell counts and hemoglobin values were lower in Prascend treated groups as compared to the control group. Other hematology parameters including hematocrit, white blood cells, absolute neutrophils, and absolute lymphocytes exhibited mild, transient decreases as compared to the control group. The hematology parameters generally decreased over the first 30 to 60 days after treatment initiation and then returned to values similar to pre-treatment levels. No treatment related alterations were identified on histopathology evaluation of bone marrow.
- Storage
-
How Supplied
Prascend Tablets are available in 1 mg strength – packaged 10 tablets per blister and 60 or 160 tablets per carton.
NDC: 0010-4489-01 – 60 tablets
NDC: 0010-4489-02 – 160 tablets
-
References
1 Orth, D.N., Holscher, M.A., Wilson, M.G., et al. (1982) Equine Cushing’s Disease: Plasma Immunoreactive Proopiolipomelanocortin Peptide and Cortisol Levels Basally and in Response to Diagnostic Tests. Endocrinology. 110(4):1430-41
2 Wright A, Gehring R, Coetzee H (2008.) Pharmacokinetics of pergolide in normal mares. American College of Veterinary Internal Medicine Forum, Abstract #36, San Antonio, TX.
-
SPL UNCLASSIFIED SECTION
Manufactured for:
Boehringer Ingelheim Vetmedica, Inc.
St. Joseph, MO 64506 U.S.A.Made in Japan and packaged in Germany.
Prascend is a registered trademark of Boehringer Ingelheim Vetmedica GmbH used under license.
© 2016 Boehringer Ingelheim Vetmedica, Inc. All Rights Reserved.
448901-01
Revised 07/2016
- Principal Display Panel 10 – Tablet Blister Pack
- Principal Display Panel - 60 – Tablet Display Carton
-
INGREDIENTS AND APPEARANCE
PRASCEND
pergolide tabletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0010-4489 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PERGOLIDE MESYLATE (UNII: 55B9HQY616) (PERGOLIDE - UNII:24MJ822NZ9) PERGOLIDE 1 mg Product Characteristics Color RED (Light red) Score 2 pieces Shape RECTANGLE Size 12mm Flavor Imprint Code PRD Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0010-4489-01 6 in 1 CARTON 1 10 in 1 BLISTER PACK 2 NDC: 0010-4489-02 16 in 1 CARTON 2 10 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141331 10/25/2011 Labeler - Boehringer Ingelheim Vetmedica, Inc. (007134091) Registrant - Boehringer Ingelheim Vetmedica, Inc. (007134091)
Trademark Results [Prascend]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PRASCEND 78964991 not registered Dead/Abandoned |
Eli Lilly and Company 2006-08-31 |
 PRASCEND 77309144 3702241 Live/Registered |
BOEHRINGER INGELHEIM VETMEDICA GMBH 2007-10-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.